Volume 19, No.1 Pages 2 - 6
1. 最近の研究から/FROM LATEST RESEARCH
Anomalous Ground State of the Electrons in Nano-confined Water
[1]Department of Chemistry, University of Michigan, [2]Physics Department, University of Houston, [3]Research & Utilization Division, JASRI, [4]Department of Chemical and Biomolecular Engineering, University of Tennessee
- Abstract
- Water under nano-confinement is known to exhibit different properties from that of bulk water. Recent neutron scattering investigations showed that the proton momentum distribution is qualitatively different from that of the bulk water for water confined on a scale of 20 Å. Since the confining potential for the protons is due to the electrons, the electronic ground state of nano-confined water should also be anomalous. X-ray Compton scattering, which probes the ground state of a system, was utilized to understand the ground state configuration of the valence electrons of a particular nano-confined water system, Nafion, a proton exchange membrane (PEM) used in fuel cells. The results showed, for the first time, that the electrons are in a different quantum state from that of bulk water. This difference cannot be explained by empirical models based on weakly interacting molecules. The separation of elements of biological cells is about 20 Å, therefore we would expect the functioning of the cells to be determined by the properties of this nano-confined state.
1. Introduction
Nano-confined water, confined on a scale of 20 Å, is known to exhibit equilibrium and dynamical properties that are different from that of bulk water[1][1] W. H. Thompson, Annu. Rev. Phys. Chem. 62, 599 (2011).. These properties have been theoretically interpreted primarily on the basis of empirical models of water, which assume a model of weakly interacting molecules. Previous investigations have shown that this model is inadequate to describe the proton momentum distribution in water confined in carbon nanotubes, xerogel, and Nafion[2][2] G. Reiter, A. Kolesnikov, S. Paddison, P. Platzman, A. Moravsky, M. Adams and J. Mayers, Physical Review B 85, 045403 (2012).. Indeed, it is even quantitatively unable to explain the momentum distribution in bulk water at standard temperature and pressure (STP)[3][3] C. J. Burnham, T. Hayashi, R. L. Napoleon, T. Keyes, S. Mukamel and G. Reiter, J. Chem. Phys. 135, 144502 (2011).. These earlier investigations have suggested that the properties of the hydrogen bond network are responsible for the differences, where the electronic overlap between acceptor oxygens and donor protons in the hydrogen bond is sufficiently strong that the network as a whole can respond in ways that are not possible for a collection of molecules interacting weakly electrostatically. Beyond our theoretical speculations, however, the fact that the momentum distributions in the confined systems are so different from those of bulk water means that the part of the many-body Born-Oppenheimer surface sampled by the protons that lead to these momentum distributions must be qualitatively different from that of a proton in a covalent bond, weakly interacting electrostatically with an acceptor oxygen. Hence, the spatial distribution of valence electrons in the hydrogen bond network in nano-confined water system would also be qualitatively different from that of bulk water.
These changes in the spatial distribution of valence electrons of bulk water will be reflected in the momentum distribution of the electrons, and can be directly observed utilizing x-ray Compton scattering, an inelastic x-ray scattering process at large energy and momentum transfers, probing the electronic ground state of the target system. We show here that these predicted changes can indeed be observed, though it is not possible to relate the measured electron momentum distribution differences directly to Born-Oppenheimer potentials, as was done for the proton momentum distribution[4][4] D. Homouz, G. Reiter, J. Eckert, J. Mayers and R. Blinc, Phys. Rev. Lett. 98, 115502 (2007).. We observe that the differences are much larger than those produced by disordering the hydrogen bond of a pair of water molecules, as happens as water is heated. The difference in bond disorder between water confined in Nafion, and bulk water, is seventeen times larger than the difference between bulk water just above the freezing point, and bulk water just below the boiling point, the latter being a difference easily measured with x-ray Compton scattering. We conclude that this change of the electron momentum distribution observed is not possible within the model of weakly interacting molecules, and requires the redistribution of electrons through the hydrogen bond network. With this approach, we also present here reinterpretation of fluorescence[5][5] D. E. Moilanen, D. B. Spry and M. D. Fayer, Langmuir 24, 3690 (2008). and pump probe experiments[6][6] K. J. Tielrooij, M. J. Cox and H. J. Bakke, CPPC 10, 249 (2009). performed earlier by others, to support this conclusion.
______________________________
* To whom Correspondence should be addressed: debani@umich.edu
2. Experimental Methods
We restrict our discussion here to water confined in two types of Nafion, Nafion 1120 and Dow 858, where Nafion is a perfluorosulfonic acid membrane. These are the same samples as those used in the neutron Compton scattering measurements, in order to eliminate sample variability[7][7] K. D. Kreuer, M. Schuster, B. Obliers, O. Diat, U. Traub, A. Fuchs, U. Klock, S. Paddison and J. Maier, Journal of Power Sources 178, 499 (2008).. These are ionomers with hydrophobic poly- (tetra-fluoroethylene) (PTFE) backbones and randomly pendant perfluoroether side chains terminating with sulfonic acids. The ionomers when hydrated exhibits a nano-phase separated morphology where the water and ions exist in domains which are only a few nanometers in diameter surrounded by the backbones[8, 9][8] D. Wu, S. J. Paddison and J. A. Elliott, Macromolecules 42, 3358 (2009).
[9] D. Wu, S. J. Paddison and J. A. Elliott, Energy Environ. Sci. 1, 284 (2008). (Fig. 1). The sulfonic acid group (-SO3H) donates protons to the water, when there is sufficient water, making them very good proton conductors, and hence popularly used as the electrolyte in commercial fuel cells. The samples prepared were cleaned in nitric acid, and loaded with water by equilibration with vapor of a LiCl salt solution, of known concentration for two weeks. The concentration of water relative to the number of sulfonyl groups, λ, was 14, same as it was in the neutron experiments. The samples used were first sealed in the x-ray sample cells while in contact with the vapor to avoid any loss of water in the atmosphere. The samples were carefully monitored, by weighing them before and after the measurements, which showed no significant weight change. A dry sample was prepared by leaving the sample in vacuum for five days at room temperature. To estimate the true background contribution, measurement of the dry sample (Fig. 2) was performed as background before filling the samples with water. The signal from the dry Nafion (for both Nafion 1120 and DOW 858) was subtracted from that of the hydrated sample to obtain the signal for the confined water. The experiments were performed at the BL08W, high energy inelastic scattering beam line at SPring-8. The measurements were performed at an incident energy of 182 keV, at a scattering angle of 178.3o and the scattered photons were collected utilizing a ten-element Ge solid-state detector. For the measurement, the samples were confined in an Al sample-holder of 3 mm thick, with Kapton windows (~10 µm thick) used as the x-ray window and the sample was placed in a vacuum chamber to minimize the background due to scattering from air. All the measurements were performed at room temperature and as large statistics are necessary to observe the small changes between the confined and the bulk water, the data were constantly monitored by checking for consistency, for variation larger than the statistical accuracy, after every 12 minutes. For good statistics, the total counts in each raw Compton profile (CP), under the Compton peak was more than 1 × 109 counts. The measured CP's were then corrected for the necessary energy dependent corrections, absorption, detector efficiency, and multiple scattering, before converting to the momentum scale utilizing the relativistic cross-section correction. The CP's were then binned at steps of 0.1 a.u. and the positive and the negative momentum sides were folded to increase the statistical accuracy. As a part of the comparison with the bulk water, we also performed measurements of a sample in the same sample holder containing bulk deionized (DI) water (Fig. 2), measured under the same experimental conditions.
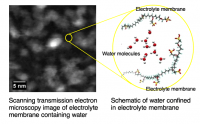
Figure 1: Scanning transmission electron microscopy (STEM) image of the wet Nafion 1120 containing water. The schematic on the right shows how the water is nano-confined in the backbone of the Nafion 1120 PEM.
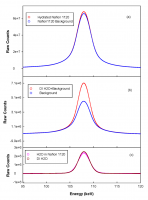
Figure 2: Comparison of CP of (a) Hydrated Nafion 1120 (red), and background from dry Nafion 1120 (blue); (b) DI water with background (red) and background only (blue); (c) water in Nafion 1120, obtained from subtracting the CP of background from CP of hydrated Nafion 1120 (pink), DI water, obtained from subtracting the CP of background from CP of DI water with background (dark red).
For the data analysis, the valence-electron CP's of both the confined and bulk samples were obtained by subtracting the theoretical core electron profile contribution from the experimental profiles. The theoretical core-profile contribution was taken based on the free-atom Hartree-Fock simulations[10][10] F. Biggs, L. B. Mendelsohn and J. B. Mann, At. Data Nucl. Data Tables 16, 201 (1975)., where we have treated oxygen (1s)2 as the core electrons, and finally as we are comparing the subtle shape changes of the CP between the bulk and the nano-confined water, the CP's were carefully again renormalized to 8 valence electrons, for proper comparison. The bulk water profile was in good agreement with an earlier theoretical model[11][11] C. Bellin, B. Barbiellini, S. Klotz, T. Buslaps, G. Rousse, T. Strassle and A. Shukla, Physical Review B 83, 094117 (2011). and is shown in the inset of Fig. 3.
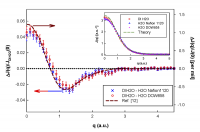
Figure 3: The difference CP of Nafion 1120 and Dow 858 subtracted from the CP of bulk water. The red dashed line[12][12] K. Nygård, M. Hakala, T. Pylkkänen, S. Manninen, T. Buslaps, M. Itou, A. Andrejczuk, Y. Sakurai, M. Odelius and K. Hämäläinen, J. Chem. Phys. 126, 154508 (2007). is a fit to the difference (H2O-D2O) between H2O and D2O, rescaled to fit our data; a rescaling by a factor of 46 is needed. The inset shows the experimental CP for DI water, confined water in two types of Nafion (Nafion 1120 and DOW 858) and a previous reported[11][11] C. Bellin, B. Barbiellini, S. Klotz, T. Buslaps, G. Rousse, T. Strassle and A. Shukla, Physical Review B 83, 094117 (2011). theoretical CP (green dashed line) of isolated water molecule.
3. Results and Discussions
The CP's for the two Nafion samples and bulk water are shown in the inset of Fig. 3, and as a comparison of all the results, the subtracted profile for the two Nafion samples from bulk water, has been compared with a calculation by Nygård et. al.[12][12] K. Nygård, M. Hakala, T. Pylkkänen, S. Manninen, T. Buslaps, M. Itou, A. Andrejczuk, Y. Sakurai, M. Odelius and K. Hämäläinen, J. Chem. Phys. 126, 154508 (2007). of the difference between the CP's of H2O and D2O. The comparison uses a dimer approximation with the distribution of angles and bond lengths in bulk water inferred from NMR measurements. In this calculation, it was assumed that all the reordering of the electron distribution is due to changes in the configuration of the hydrogen bond between a single donor and single acceptor water molecule. It is important to note here, that this approximation has been used to satisfactorily fit a series of experimental CP's for bulk water between temperatures 5˚C and 90˚C[13][13] M. Hakala, K. Nygård, S. Manninen, S. Huotari, T. Buslaps, A. Nilsson, L. G. M. Pettersson and K. Hämäläinen, J. Chem. Phys. 125, 084504 (2006)..
As is seen clearly from our experimental results (Fig. 3), this previous theoretical model used for explaining the changes in bulk water is inadequate. The maximum amplitude of [∆J(0)/J(0)], the fractional change in the CP at zero momentum (q=0), for confined water is 0.05. The maximum difference in [∆J(0)/J(0)] between water at 5˚C and 90˚C is only 0.003[13][13] M. Hakala, K. Nygård, S. Manninen, S. Huotari, T. Buslaps, A. Nilsson, L. G. M. Pettersson and K. Hämäläinen, J. Chem. Phys. 125, 084504 (2006).. With this direct comparison, where we consider the change is the measure of the disorder of the hydrogen bond network, the disordering of the hydrogen bond network due to the confinement is 17 times that produced by the thermal disordering in going from just above freezing to just below boiling, and 46 times the difference between H2O and D2O at comparable temperatures. This is not entirely unexpected, as the proton momentum distribution for the two Nafion samples compared to that of water oscillations are indicative of the proton being coherently distributed in a double well with a separation of the wells on the order of 0.3 Å[2][2] G. Reiter, A. Kolesnikov, S. Paddison, P. Platzman, A. Moravsky, M. Adams and J. Mayers, Physical Review B 85, 045403 (2012).. The kinetic energy has gone up because each of these wells is more tightly binding the proton than the covalent bond of the isolated water molecule. The kinetic energy is 245 meV and 268 meV for the Nafion and Dow samples respectively, compared to 148 meV for bulk water at room temperature. The change in kinetic energy in going from 5˚C to 90˚C for bulk waters is only 0.5 meV.
The direction of the change for the electron CP is consistent with the tighter binding of the proton, which we would expect to require a greater localization of the valence electrons in the vicinity of the proton, and hence a broader CP, as observed. It is conceivable that the changes we are seeing here are the result of changes in the electron distribution in the ionomer due to the morphological changes that occur as the water swells the dry Nafion. We think this is unlikely, since the C-F bonds that describe the ionomer (Teflon) are unlikely to be affected significantly by the physical displacements of the ionomer or by interaction with the water molecules. Furthermore, the two different samples used here have different morphologies, due to the difference in the size of the side chains containing the sulfonyl groups. They yield, nevertheless, very similar subtracted CP's (between the confined water in Nafion and bulk water), within the error bars.
It might also be thought that the presence of the extra proton, donated by the sulfonyl groups (and responsible for the high conductivity of Nafion) is changing the electron distribution in its vicinity sufficiently to make up the large difference in the subtracted profile. Beyond the fact that there is only 1 proton in 28 which is free, we have the evidence of experiments and calculations on LiCl, which is known to strongly disorder the hydrogen bond network, that the changes of (1/2) [∆J(0)/J(0)], from bulk water at similar concentrations of Li+, are of the order of 0.005. The lack of a dramatic effect in the electron Compton scattering is mirrored in the neutron Compton scattering, where only small deviations of the momentum distribution from that of bulk water are seen at these concentrations[14][14] G. Reiter, J. Mayers and T. Abdul-Redah, Physica B 385-386, 234 (2006).. Hence we conclude that the quantum ground state of the electron-proton system when the hydrogen bond network is disordered by nano-confinement is qualitatively different from the ground state of a weakly interacting collection of molecules.
Some further support for our explanation comes from excited state proton transfer measurements of a fluorescent molecule, 8-hy- droxypyrene-1,3,6-trisulfonate (HPTS), utilized as a probe for the proton dynamics[15][15] D. B. Spry, A. Goun, K. Glusac, D. E. Moilanen and M. D. Fayer, JACS 129, 8123 (2007).. The molecule tends to stay in the middle of the water filled regions in the Nafion. The electronic state is excited by a laser pulse, which leads to the proton in the OH group of the molecule being ionized. The recombination time depends on the transport processes affecting the now free proton. Assuming a diffusion process for that transport leads to a t-1.5 dependence of the rate of recombination for long recombination times. This is what has been observed in bulk water, while in Nafion the observed rate is t-0.8. Evidently, the transport of the proton is not a diffusion process, it would be a diffusion process for long times as long as the "jumps" of the proton from one location to another are determined by the local conditions in the vicinity of the proton as it moves from one equivalent position to another, and there is no memory of where the proton came from on the next jump. One or both of these conditions must be violated in the transport of protons in Nafion. The Grothuus mechanism, in which the proton that is moving changes identity, but otherwise moves from site to site between the water molecules, does lead to a diffusion process. The slowing down of the transport of the protons that have been photo excited in the confined water cannot be due to the protons reflected off the surroundings, as these would only speed up the recombination of the HPTS ion with the dissociated protons. To change the exponent requires some collective response of the hydrogen bond electron-proton network to the motion of the proton. That this response is a property of confined water, and not some peculiarity of Nafion is demonstrated by the fact that the same behavior is seen in reverse micelles of comparable size to the pores of Nafion[15][15] D. B. Spry, A. Goun, K. Glusac, D. E. Moilanen and M. D. Fayer, JACS 129, 8123 (2007)..
Direct confirmation that the electronic state in nano-confined water differs significantly from that in bulk water is also found in pump-probe experiments in which the excitation of the HPTS is observed to decay on a rapid time scale, due to direct de-excitation of the electronic state without the return of the ionized proton[6][6] K. J. Tielrooij, M. J. Cox and H. J. Bakke, CPPC 10, 249 (2009)., which does not happen in bulk water.
The characteristic scale at which the nano-confined ground state appears is 20 Å, the scale of the distance between elements of biological cells. It would be remarkable if evolution had such a state available and didn't use it. We expect the quantum properties of this state have a profound effect on the functioning of cells.
Acknowledgements
G. Reiter's work was supported by the DOE, Office of Basic Energy Sciences under Contract No. DE-FG02-08ER46486. G. Reiter and Aniruddha Deb would like to thank Phil Platzman, now deceased, for many rewarding conversations and the inspiration to look at x-ray Compton scattering for a signature of the new quantum state. S. Paddison acknowledges support by the U.S. Army Research Office under Contract Number W911NF-07-1-0085. These experiments were performed with approval of the Japan Synchrotron Radiation Research Institute (JASRI)/SPring-8, Proposal No. 2011A1074.
References
[1] W. H. Thompson, Annu. Rev. Phys. Chem. 62, 599 (2011).
[2] G. Reiter, A. Kolesnikov, S. Paddison, P. Platzman, A. Moravsky, M. Adams and J. Mayers, Physical Review B 85, 045403 (2012).
[3] C. J. Burnham, T. Hayashi, R. L. Napoleon, T. Keyes, S. Mukamel and G. Reiter, J. Chem. Phys. 135, 144502 (2011).
[4] D. Homouz, G. Reiter, J. Eckert, J. Mayers and R. Blinc, Phys. Rev. Lett. 98, 115502 (2007).
[5] D. E. Moilanen, D. B. Spry and M. D. Fayer, Langmuir 24, 3690 (2008).
[6] K. J. Tielrooij, M. J. Cox and H. J. Bakke, CPPC 10, 249 (2009).
[7] K. D. Kreuer, M. Schuster, B. Obliers, O. Diat, U. Traub, A. Fuchs, U. Klock, S. Paddison and J. Maier, Journal of Power Sources 178, 499 (2008).
[8] D. Wu, S. J. Paddison and J. A. Elliott, Macromolecules 42, 3358 (2009).
[9] D. Wu, S. J. Paddison and J. A. Elliott, Energy Environ. Sci. 1, 284 (2008).
[10] F. Biggs, L. B. Mendelsohn and J. B. Mann, At. Data Nucl. Data Tables 16, 201 (1975).
[11] C. Bellin, B. Barbiellini, S. Klotz, T. Buslaps, G. Rousse, T. Strassle and A. Shukla, Physical Review B 83, 094117 (2011).
[12] K. Nygård, M. Hakala, T. Pylkkänen, S. Manninen, T. Buslaps, M. Itou, A. Andrejczuk, Y. Sakurai, M. Odelius and K. Hämäläinen, J. Chem. Phys. 126, 154508 (2007).
[13] M. Hakala, K. Nygård, S. Manninen, S. Huotari, T. Buslaps, A. Nilsson, L. G. M. Pettersson and K. Hämäläinen, J. Chem. Phys. 125, 084504 (2006).
[14] G. Reiter, J. Mayers and T. Abdul-Redah, Physica B 385-386, 234 (2006).
[15] D. B. Spry, A. Goun, K. Glusac, D. E. Moilanen and M. D. Fayer, JACS 129, 8123 (2007).