Volume 18, No.3 Pages 214 - 222
1. 最近の研究から/FROM LATEST RESEARCH
Long-term Proposal Report: XMCD Study of Capped ZnO Nanoparticles: The Quest of the Origin of Magnetism
[1]Instituto de Ciencia de Materiales de Aragón, CSIC-Un. Zaragoza, [2]Dpto. Física de Materiales, Un. Complutense de Madrid, [3]Instituto de Ciencia de Materiales de Madrid, CSIC, [4]Instituto de Cerámica y Vidrio, CSIC
- Abstract
- Discoveries of room-temperature ferromagnetism (RTFM) in diluted magnetic oxides and semiconductors hold great promise in future spintronics technologies. Unfortunately, this ferromagnetism remains poorly understood and the debate concerning the nature of ferromagnetism in semiconducting oxides is still open. Here, we demonstrate by using X-ray absorption (XAS) and X-ray magnetic circular dichroism (XMCD) the intrinsic occurrence of RTFM in these systems and point out that it is not related to the metallic cation but it relays on the conduction band of the semiconductor. We present here direct experimental evidence of the magnetic polarization of Zn atoms in ZnO nanoparticles capped with different organic molecules and on ZnS/ZnO heterostructures. The analysis of both XAS and XMCD spectra indicates the formation of a well defined interface between ZnO and the capping molecule in which the exotic magnetism resides. The occurrence of ferromagnetism does not critically depend on the details of the synthesis but on the formation of a pristine ZnS/ZnO interface. These results provide an avenue to explain the surprising results found in this field, sometimes seemingly irreconcilable, ending the longstanding controversy about the existence of intrinsic RTFM in ZnO-based systems. Moreover, they provide new insights to finally establish the mechanism that sets on the ferromagnetic order in these systems and bringing support to this new route for room-temperature semiconductor spintronics.
1. Introduction
One of the main goals of material science nowadays is the development of multifunctional materials combining properties that do not stand together in traditional materials. For instance, the coexistence of semiconductor properties (basis of the microprocessors) and room temperature ferromagnetism (as non volatile memories) in a single material will push the development of new and optoelectronic devices with higher reliability and lower power consumption. In the past thirteen years, a great deal of effort has been put into the investigation of the mechanisms behind the ferromagnetism in dilute magnetic semiconductors (DMSs), dilute magnetic oxides (DMOs) and even for materials containing no transition-metal impurities, for which a ferromagnetic response persisting up to above room temperature (RTFM) has been reported even when no ferromagnetism was expected at any temperature1-6[1] Chambers, S. A. Ferromagnetism in doped thin-film oxide and nitride semiconductors and dielectrics. Surf. Sci. Rep. 2006; 61: 345–381.
[2] Pearton, S. J.; Heo, W. H.; Ivill, M.; Norton, D. P.; Steiner, T. Semicond. Sci. Technol. 2004; 19, R59–R74.
[3] Sato, K.; Katayama-Yoshida, H. Ferromagnetism in a transition metal atom doped ZnO. Physica E 2001; 10: 251–255.
[4] Matsumoto, Y.; Murakami, M.; Shono, T.; Hasegawa, T.; Fukumura, T.; Kawasaki, M.; Ahmet, P.; Chikyow, T.; Koshihara, S.; Koinuma, H. Room-Temperature Ferromagnetism in Transparent Transition Metal-Doped Titanium Dioxide. Science 2001; 291: 854–856.
[5] Coey, J. M. D.; Chambers, S. A. Oxide Dilute Magnetic Semiconductors - Fact or Fiction? MRS Bulletin 2008; 33: 1053–1058.
[6] Kittilstved, K. R.; Liu, W. K.; Gamelin, D. R. Electronic structure origins of polarity-dependent high-TC ferromagnetism in oxide-diluted magnetic semiconductors. Nature Mater. 2006; 5: 291–297..
Despite some initial promising results, it is not clear if those materials are intrinsically ferromagnetic and nowadays the situation is confusing. Still, after more than ten years of intense research, the origin of ferromagnetism in these systems remains a controversial issue from both theoretical7[7] Zunger, A.; Lany, S.; Raebiger, H. The quest for dilute ferromagnetism in semiconductors: Guides and misguides by theory. Physics 2010; 3: 53. and experimental points of view1,5,8,9[1] Chambers, S. A. Ferromagnetism in doped thin-film oxide and nitride semiconductors and dielectrics. Surf. Sci. Rep. 2006; 61: 345–381.
[5] Coey, J. M. D.; Chambers, S. A. Oxide Dilute Magnetic Semiconductors - Fact or Fiction? MRS Bulletin 2008; 33: 1053–1058.
[8] Chambers, S. Is it really intrinsic ferromagnetism? Nat. Mater. 2010; 9: 956–957.
[9] Lawes, G.; Risbud, A. S.; Ramíırez, A. P.; Seshadri, R. Absence of ferromagnetism in Co and Mn substituted polycrystalline ZnO. Phys. Rev. B 2005; 71: 045201/1–045201/5.. Most reported evidences of room-temperature ferromagnetism are based on macroscopic magnetometry results. Unfortunately, in many cases no exhaustive characterization of the materials has been made at the microscopic level so that the ferromagnetism might be likely due to extrinsic effects as magnetic contamination or magnetic secondary-phase formation1,5,9,10[1] Chambers, S. A. Ferromagnetism in doped thin-film oxide and nitride semiconductors and dielectrics. Surf. Sci. Rep. 2006; 61: 345–381.
[5] Coey, J. M. D.; Chambers, S. A. Oxide Dilute Magnetic Semiconductors - Fact or Fiction? MRS Bulletin 2008; 33: 1053–1058.
[9] Lawes, G.; Risbud, A. S.; Ramíırez, A. P.; Seshadri, R. Absence of ferromagnetism in Co and Mn substituted polycrystalline ZnO. Phys. Rev. B 2005; 71: 045201/1–045201/5.
[10] García, M. A.; Pinel, E. F.; de la Venta, J.; Quesada, A.; Bouzas, V.; Fernández, J. L.; Romero, J. L.; Martín-González, M. S.; Costa-Krämer, J. L. Sources of experimental errors in the observation of nanoscale magnetism. J. Appl. Phys. 2009; 105: 013925/1–7.. This points the need of using more sophisticated characterization tools able to provide atom-specific magnetic properties and a detailed view of the local structure of the systems under study. The accumulated experience so far dictates the need of using atom-specific structural and magnetic probes as X-ray magnetic circular dichroism (XMCD) and X-ray absorption spectroscopy (XAS) to elucidate the intrinsic nature of the RTFM behaviour11-12[11] Barla, A.; Schmerber, G.; Beaurepaire, E.; Dinia, A.; Bieber, H.; Colis, S.; Scheurer, F.; Kappler, J. P.; Imperia, P.; Nolting, F.; Wilhelm, F.; Rogalev, A.; Müller, D.; Grob, J. J. Paramagnetism of the Co sublattice in ferromagnetic Zn1-xCoxO films. Phys. Rev. B 2007; 76: 125201/1–5.
[12] Céspedes, E.; Laguna-Marco, M. A.; Jiménez-Villacorta, F.; Chaboy, J.; Boada, R.; Guglieri, C.; Andrés, A. de.; Prieto, C. On the Origin of the Magnetism of Mn-Zn-O Systems: Structural, Electronic, and Magnetic Study of Exotic MnO2-δ/ZnO Thin Films. The Journal of Physical Chemistry C 2011; 115: 24092–24101..
Application of these advanced tools to the study of Co:ZnO DMOs reveals11[11] Barla, A.; Schmerber, G.; Beaurepaire, E.; Dinia, A.; Bieber, H.; Colis, S.; Scheurer, F.; Kappler, J. P.; Imperia, P.; Nolting, F.; Wilhelm, F.; Rogalev, A.; Müller, D.; Grob, J. J. Paramagnetism of the Co sublattice in ferromagnetic Zn1-xCoxO films. Phys. Rev. B 2007; 76: 125201/1–5. that the RTFM found in bulk magnetization measurements was not due to Co, whose 3d states show paramagnetic behaviour according to XMCD. Similar results were found in the case of Cu-doped ZnO thin films, which display robust room-temperature ferromagnetic signatures using bulk magnetization probes. Keavney and coworkers probed the XMCD at the Cu(3d), O(2p), and Zn(3d) states. They found no dichroic signal consistent with ferromagnetism originating from any of these states: only a paramagnetic component was detected at the Cu(3d), and no magnetic signal in the O or Zn13[13] Keavney, D. J.; Buchholz, D. B.; Ma, Q.; Chang, R. P. H. Where does the spin reside in ferromagnetic Cu-doped ZnO? Appl. Phys. Lett. 2007; 91: 012501/1–3.. The experimental findings demonstrating that the 3d electronic shells of the cations in these DMOs do not carry any measurable ferromagnetic moment, contrary to what is usually assumed in the theoretical models proposed, go in line with Coey’s wondering regarding “if the dilute doping of the oxides with magnetic cations may be something of a smokescreen as far as the magnetism is concerned.”14[14] Coey, J. M. D. Dilute magnetic oxides. Current opinion in Solid State and. Mat. Sci. 2006; 10, 83–92. This led to a renewed interest in the field after the observation of ferromagnetism in semiconductor and insulating oxide nanostructures without any doping despite the diamagnetic character of the material in bulk6,15-17[6] Kittilstved, K. R.; Liu, W. K.; Gamelin, D. R. Electronic structure origins of polarity-dependent high-TC ferromagnetism in oxide-diluted magnetic semiconductors. Nature Mater. 2006; 5: 291–297.
[15] Carmeli, I.; Leitus, G.; Naaman, R.; Reich, S.; Vager, Z. Magnetism induced by the organization of self-assembled monolayers. J. Chem. Phys. 2003; 118: 10372–10375.
[16] Coey, J. M. D.; Venkatesan, M.; Fitzgerald, C. B. Donor impurity band exchange in dilute ferromagnetic oxides. Nature Mater. 2005; 4: 173–179.
[17] García, M. A.; Merino, J. M.; Fernández Pinel, E.; Quesada, A.; de la Venta, J.; Ruíz-González, M. L.; Castro, G. R.; Crespo, P.; Llopis, J.; González-Calbet, J. M.; Hernando, A. Magnetic Properties of ZnO Nanoparticles. Nano Letters 2007; 7: 1489–1494..
In the particular case of ZnO nanoparticles (NPs), it was demonstrated that it is possible to induce room-temperature ferromagnetic-like (FML) behaviour in these ZnO nanoparticles by capping them with different molecules17[17] García, M. A.; Merino, J. M.; Fernández Pinel, E.; Quesada, A.; de la Venta, J.; Ruíz-González, M. L.; Castro, G. R.; Crespo, P.; Llopis, J.; González-Calbet, J. M.; Hernando, A. Magnetic Properties of ZnO Nanoparticles. Nano Letters 2007; 7: 1489–1494.. These results were interpreted in terms of a correlation between the charge transfer produced by the surface bond and the magnetic moment, proposing that their magnetism is related to the modification of the electronic structure of the nanoparticles due to the bonds with the molecules. By this reason, the study of the appearance of magnetism in nanoparticles of materials without doping that are non magnetic in bulk is essential to establish on firmer grounds the intrinsic nature of this new high-temperature magnetism. Indeed, elucidation of its origins has been cited as among the most important problems in magnetism to have emerged in several years16,18[16] Coey, J. M. D.; Venkatesan, M.; Fitzgerald, C. B. Donor impurity band exchange in dilute ferromagnetic oxides. Nature Mater. 2005; 4: 173–179.
[18] T. Dietl, Dilute magnetic semiconductors: Functional ferromagnets. Nat. Mater. 2003; 2, 646-648..
Aimed to this we started this long-term study focussed on the magnetic behaviour of ZnO nanoparticles capped with different organic molecules, showing magnetic properties ranging from pure diamagnetism to the appearance of a ferromagnetic-like contribution. Our main objective is to provide a full characterization of this new magnetic behaviour, i.e., how the alteration of the electronic structure of the semiconductor by capping with certain molecules can lead to the appearance of RT ferromagnetic behaviour even in absence of magnetic ions.
To this end, we have performed a systematic X-ray magnetic circular dichroism (XMCD) study at the K-edge of Zn in ZnO nanoparticles. The XMCD spectra have been recorded as a function of the applied magnetic field and temperature on different ZnO-based systems that have been tailored by varying: i) the capping molecules, ii) the size of the nanoparticle, and iii) the nature (order-disorder) of the interface formed in the bonding between the NP. This long-term proposal was intended to solve the following questions: i) which are the atoms responsible for the observed magnetism?, and ii) where (ZnO particle, surface, bonded interface) is this magnetism located?
2. Experimental Methods
ZnO NPs were prepared by sol-gel and subsequently capped with three different organic molecules: tryoctylphosphine (TOPO), dodecylamine (AMINE), and dodecanethiol (THIOL), which bond to the particle surface through an O, N, and S atom, respectively. The samples were prepared in several series, each series using the same starting solution, by varying the time at which the capping agent is added. In order to investigate the role of ZnO/ZnS interfaces, two ZnO-ZnS multilayers, labeled (ZnO4nm/ZnS4nm)10 and ZnO2nm/ZnS2nm)20. Finally, a Zn-O-S thin film (ZnS/ZnO-50/50) was prepared by RF co-sputtering. A detailed description of the samples and preparation method as well their characterization (XRD, HRTEM, EDX, SQUID magnetometry) can be found elsewhere19-21[19] Chaboy, J.; Boada, R.; Piquer, C.; Laguna-Marco, M. A.; García-Hernández, M.; Carmona, N.; Llopis, J.; Ruíz-González, M. L.; González-Calbet, J.; Fernández, J. F.; García, M. A. Evidence of intrinsic magnetism in capped ZnO nanoparticles. Phys. Rev. B 2010; 82: 064411/1–9.
[20] Guglieri, C.; Laguna-Marco, M. A.; García, M. A.; Carmona, N.; Céspedes, E.; García-Hernández, M.; Espinosa, A.; Chaboy, J. XMCD proof of ferromagnetic behaviour in ZnO nanoparticles. J. Phys. Chem. C 2012; 116: 6608–6614.
[21] Guglieri, C.; Espinosa, A.; Carmona, N.; Laguna-Marco, M. A.; Céspedes, E.; Ruíz-González, M. L.; González-Calbet, M.; García-Hernández, M.; García, M. A.; Chaboy, J. Relationship between the magnetic properties and the formation of a ZnS/ZnO interface in S-capped ZnO nanoparticles and ZnS-ZnO thin films. J. Phys. Chem. C 2013; 117: 12199–12209.. Zn K-edge XAS and XMCD experiments were performed at the beamline BL39XU of the SPring-8 Facility. The experiments were carried out at fixed temperatures, ranging from T = 5 K to ambient and under an applied magnetic field of up 10 T. XMCD spectra of the nanoparticles and bulk ZnO and ZnS reference samples were recorded in the transmission mode by using the helicity-modulation technique. We have verified in all of the cases that the recorded spectra are not affected by the long beam-exposure time needed. Moreover, these measurements have been accumulated through a three year period on the same samples and specimens. No modification of both XAS and XMCD spectra has been found on the same specimens measured at the initial run and along a three years period, either in different specimens prepared from the same sample, which proves the stability of the synthesized samples.

Fig. 1: (a) Magnetization vs applied magnetic field curves of the dodecanethiol-capped ZnO NPs (12C-2 series) measured at T = 250 K, after subtracting the diamagnetic linear background. Samples are labeled as tadd-12-batch, where tadd is the time after adding the TMAH and before adding the organic molecule, nC (n = 4, 8 and 12) the number of carbons of the molecule, and -batch indicates the batch of synthesis (see Ref. 21 for further details). (b) Magnetization curves of the (ZnO4nm/ZnS4nm)10 thin film measured at T = 5 K and at room temperature. (c,d) The same as that described above in the case of the bulk ZnO reference and of the ZnS/ZnO-50/50 sample obtained by ZnS-ZnO cosputtering. The inset in panel c) show the same in the case of a ZnO single crystal.
3. Results
In order to investigate the origin of the ferromagnetic signals measured with the SQUID, we have performed a combined study of XAS and XMCD on the Zn K edge in the same samples. It should be noted that the element specificity of XMCD guarantees the absence of extrinsic contributions to the measured magnetic signals. Figure 2 reports the XAS and XMCD spectra recorded at the Zn L2,3 edges of the AMINE sample. No XMCD signal could be observed in the measured energy region, down to the noise level, in agreement with previous works11,13[11] Barla, A.; Schmerber, G.; Beaurepaire, E.; Dinia, A.; Bieber, H.; Colis, S.; Scheurer, F.; Kappler, J. P.; Imperia, P.; Nolting, F.; Wilhelm, F.; Rogalev, A.; Müller, D.; Grob, J. J. Paramagnetism of the Co sublattice in ferromagnetic Zn1-xCoxO films. Phys. Rev. B 2007; 76: 125201/1–5.
[13] Keavney, D. J.; Buchholz, D. B.; Ma, Q.; Chang, R. P. H. Where does the spin reside in ferromagnetic Cu-doped ZnO? Appl. Phys. Lett. 2007; 91: 012501/1–3.. In contrast, a clear XMCD signal is found at the Zn K-edge (Fig.3). These results unambiguously prove that the 3d electronic shells of Zn do not carry any measurable ferromagnetic moment and that the presence of defects and vacancies do not yield a partially unfilled magnetically polarized 3d shell. It should be noted that if the polarization of the Zn p states is due to the existence of a 3d Zn magnetic moment the XMCD effect at the Zn L2,3 edges would be well above the detection limit. This result evidences, on the one hand, the intrinsic nature of the magnetism of Zn atoms in these capped ZnO NPs. Moreover, as the x-ray absorption at the Zn K-edge probes the empty p states of Zn (actually 4sp due to hybridization) this result also indicates that the magnetic polarization of the Zn atoms takes place at the sp conduction band, i.e., it is p magnetism. Therefore, the absence of XMCD signal at the Zn L2,3 edges cannot be identified with the absence of Zn magnetization but only of 3d Zn magnetic moments. Indeed, if the observed magnetism is associated with the creation of oxygen 2p holes, the sp band of Zn should be also concerned due to the hybridization of both orbitals in ZnO. Hence, the Zn K-edge XMCD should directly reflect, in agreement with our findings, the magnetic polarization of these electronic states.
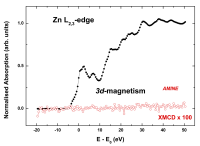
Fig. 2: XAS and XMCD Zn L2,3-edges spectra of AMINE ZnO nanoparticles recorded at T=10 K with an applied magnetic field of 5 T.
The dependence of the Zn K-edge XMCD signals as a function of the external magnetic field, XMCD(H), for THIOL and TOPO samples is shown in Figure 3. The spectral shape of the XMCD signals of the O- (TOPO) and N- (AMINE) capped nanoparticles shows a narrow positive peak in correspondence to the maximum of the XAS absorption. In contrast, this main peak broadens in the case of THIOL, appearing to be composed of two superimposed single peaks. Each of these contributions occurs close to the energy at which the main peaks (A, B) of the XAS spectra appear. The enhancement of peak A in THIOL with respect to TOPO or AMINE has been addressed to charge transfer effects involving S and Zn, as well as to the presence of vacancies and defects. However, the possibility of standing in front of a simple structural effect has not been tested. Therefore, we have investigated the possible formation of a ZnS-like phase during the capping of ZnO with dodecanethiol molecules by performing detailed ab-initio computations of the Zn K-edge XANES spectra of these ZnO nanoparticles. To this end we have considered different local structure of the Zn atoms in the inner part of the NP and those at the surface, where the bonding with the capping molecule takes place. Therefore, we have computed the Zn K-edge XANES spectrum for ZnO and for ZnO clusters in which several O atoms have been substituted by S. Finally, we have also considered the possibility that the Zn-S coordination extends beyond a single bond, leading to the formation of small Zn-S clusters at the nanoparticle-organic molecule interface. The results of the calculations demonstrate that the interaction of the ZnO NP with the THIOL does not correspond to a simple bonding effect in which one oxygen atom is substituted by a sulphur one, but the local environment of the Zn atoms is strongly modified. The best agreement with the experimental spectra (see Figure 4) is obtained by considering the formation of a ZnS/ZnO interface at the surface of the ZnO nanoparticles in which the oxygen atoms are substituted by sulfur ones, which adapt the Zn-S interatomic distance to that of ZnS22[22] Guglieri, C.; Chaboy, J. Characterization of the ZnO-ZnS Interface in THIOL-Capped ZnO Nanoparticles Exhibiting Anomalous Magnetic Properties. J. Phys. Chem. C 2010; 114: 19629–19634..
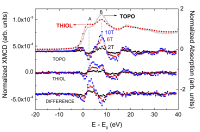
Fig. 3: Comparison of the normalized Zn K-edge XMCD spectra recorded as a function of the applied magnetic field at T = 5 K in the case of TOPO and THIOL samples, as well as their difference.
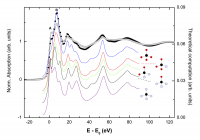
Fig. 4: Comparison of the experimental Zn K-edge XANES spectra of bulk ZnO (●) and thiol sample (○) and the theoretical signal computed for an 8 Å ZnO cluster in which the oxygen atoms (●) in the first coordination shell have been progressively substituted by S atoms (○) at the the Zn-S interatomic distance corresponding to ZnS-W.
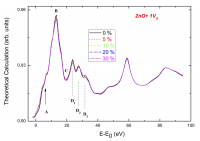
Fig. 5: Comparison of the theoretical Zn K-edge XANES spectrum of ZnO-wurtzite (black, solid line) and those calculated by considering the existence of oxygen vacancies (Vo in the first coordination shell of photoabsorbing Zn and by assuming a binomial distribution of the oxygen vacancies with different defect concentrations: no vacancies (black, solid line), 5% (red, dotted), 10% (green, dashed), 20% (blue, dotted–dashed) and 30% (cyan, short dotted–dashed–dotted)).
Finally, we have also investigated the role of vacancies and defects on the XAS spectra. Surprisingly, based on similar experimental spectra, different authors report opposing conclusions regarding the nature of defects involved in the observed RTFM. Hsu et al.23[23] Hsu, H. S.; Huang, J. C. A.; Huang, Y. H.; Liao, Y. F.; Lin, M. Z.; Lee, C. H.; Lee, J. F.; Chen, S. F.; Lai, L. Y. and Liu, C. P. Evidence of oxygen vacancy enhanced room-temperature ferromagnetism in Co-doped ZnO. Appl. Phys. Lett. 2006; 88. 242507/1-3. have concluded that oxygen vacancies enhance RT ferromagnetism in Co-doped ZnO films while, in contrast, Yan et al.24[24] Yan, W.; Sun, Z.; Liu, Q.; Li, Z.; Pan, Z.; Wang, J. and Wei, S. Zn vacancy induced room-temperature ferromagnetism in Mn-doped ZnO. Appl. Phys. Lett. 2007; 91: 062113/1-3. concluded that Zn vacancies induce RT ferromagnetism in Mn doped ZnO. This scenario is further complicated by the results of Zhang et al.25[25] Zhang, S.; Zhang, L.; Li, H.; Jiang, Z.; Chu, W.; Huang, Y.; Wang, J. and Wu, Z. Investigation of annealing-induced oxygen vacancies in the Co-doped ZnO system by Co K-edge XANES spectroscopy. J. Synchrotron Radiat. 2010; 17: 600-605. who concluded from similar data that the aforesaid oxygen vacancies are located in the second shell around the magnetic ions. These results pose serious doubts about the real capability of XAS to determine the presence of vacancies in these systems and, consequently, to shed light on the origin of the magnetism in these systems. Therefore, we have performed a detailed ab-initio study aimed at verifying the role of vacancies in modifying the XANES spectral shape of these systems. Our results demonstrated26[26] Guglieri, C.; Céspedes, E.; Prieto, C.; Chaboy, J. X-ray absorption study of the local order around Mn in Mn:ZnO thin films: the role of vacancies and structural distortions. J. Phys. Condens. Matter 23 (2011) 206006 2011; 23: 206006/1-8. that the effects induced by vacancies become undetectable in the XANES spectra when the vacancies are randomly distributed (see Figure 5).
All in all these results demonstrate the formation of a well-defined ZnS interface at the surface of the nanoparticle in which ZnS adopts the local structure of wurtzite, disregarding the possibility that the capping leads to the formation of single Zn-S bonds at the surface of the nanoparticle. Accordingly, the two-peak XMCD spectral shape observed for the S-capped sample, by contrast to the single-peak one of both N-capped and O-capped samples, is related to the magnetic polarization of Zn atoms in two well-defined ZnS and ZnO regions of the sample (see Figure 6), suggesting that the exotic magnetism observed for these NPs is related to this interface, whose details (thickness, interpenetration, etc.) should determine the particular magnetic properties of each system.
Aimed to verifying the above hypothesis we have extended the XMCD study to ZnO NPs capped no only with dodecanethiol (12 C), but also by varying the length of the carbon chain (butanethiol, 4C, and octanethiol, 8C). Moreover, we have also studied different ZnS/ZnO thin films. In all the cases, the XMCD spectra show the typical two-peak profile discussed above and, in addition, the dependence of the intensity of both peaks with the applied magnetic field is different. Having no 3d localized moment in the materials and since the observed Zn K-edge XMCD signals do not depend, both in shape and amplitude, on temperature, they can be only due to Pauli paramagnetism or to a ferro(i)- magnetic contribution. In this regard, it should be noted that the fact that the XMCD signals do not depend on temperature excludes the occurrence of a Curie−Weiss paramagnetic (CWP) Zn contribution in these orbitals. The dependence of the XMCD intensity with the applied magnetic field should be linear if it is due to Pauli paramagnetism (PP) for the studied range of magnetic fields, while it should depart from linearity if a ferromagnetic contribution is present. The expected linearity of the PP contribution has been verified in the case of reference ZnS and ZnO bulk samples. In contrast, the XMCD versus H deviates from a linear trend for H ≥ 4 T in the capped ZnO NPs (Figure 7). As expected, due to the existence of a ZnS/ZnO interface, the behaviour of THIOL is more complex and the dependence of the XMCD spectra with the applied magnetic field shows the coexistence of both types of magnetic behaviour. In particular, the low-energy peak (p1), ascribed to the ZnS-like component, exhibits a linear XMCD vs H dependence, as expected for a PP contribution, whereas the high-energy component (p2), ascribed to the ZnO, shows a saturation trend suggesting the existence of a ferromagnetic contribution. These results are in agreement with the behaviour of the integral of the XMCD signals performed in the energy range from −5.5 to 20.5 eV, as shown in the inset of Figure 7.
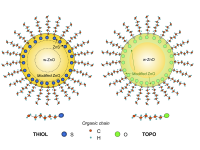
Fig. 6: Scheme of the proposed formation of an interface between the core and the surface of the ZnO nanoparticles capped with the organic molecules in the case of THIOL and TOPO samples.
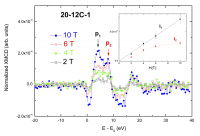
Fig. 7: Comparison of the normalized Zn K-edge XAS and XMCD spectra of dodecanethiol-capped 20-12C-1 recorded at T = 5 K and at different applied magnetic fields. The inset shows the variation with the applied magnetic field of the integrated XMCD corresponding to peaks p1 and p2 (see text for details).
All of these results are an unambiguous proof of the existence of an intrinsic FML behaviour in these capped ZnO NPs. The fact that this saturated signal is overimposed to a paramagnetic one suggests that the magnetic response is not the same for all Zn atoms in the material. It should be noted in this respect that Zn K-edge X-ray absorption measurements, XAS and XMCD, probe all Zn atoms in the material, that is, both at the core and at the surface of the NPs. The fact that the paramagnetic contribution to the XMCD signal dominates over the ferromagnetic one suggests that the latter is confined near the surface or at the interface formed between the ZnO NP and the capping molecule. Beyond the results indicating that the ZnS-like part of the sample does not contribute to FML behaviour, the detailed analysis of the XAS and XMCD spectra20[20] Guglieri, C.; Laguna-Marco, M. A.; García, M. A.; Carmona, N.; Céspedes, E.; García-Hernández, M.; Espinosa, A.; Chaboy, J. XMCD proof of ferromagnetic behaviour in ZnO nanoparticles. J. Phys. Chem. C 2012; 116: 6608–6614. indicate that the FML XMCD signal stems from a ~ 5 to 15% of the total amount of Zn atoms in the material. Taking into account that the size of the NPs is ~ 20 nm, we estimate that the FML behavior arises from a 3 to 8 Å thickness region. This has been further verified in the case of ZnO/ZnS multilayers in which the thickness of each ZnS and ZnO layer is 2 and 4 nm. According to our hypothesis the bulk-like ZnO and ZnS contribution to both the XANES and XMCD and, consequently, the paramagnetic component associated with the core should decrease, whereas the FML contribution of the interface should maximize. Therefore, the XMCD spectra will be directly comparable to the FML contribution extracted from the XMCD of the capped NPs after renormalization to the relative percentage of the interface. The result of the comparison, reported in Ref. 20, is in agreement with our starting hypothesis.
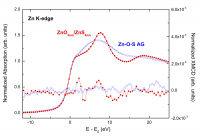
Fig. 8: Comparison of the XMCD spectra recorded at H = 10 T on the (ZnO4nm/ZnS4nm)10 heterostructure and on the ZnS-ZnO cosputtered sample.
We have finally studied another parameter playing a significant role in the description of the synthesized nanoparticles, i.e. the degree of crystallinity. In this regard, the degree of definition of peaks A and B as well as the modification affecting the three-peak structure just above the main absorption peak (see Figure 5) is of particular significance. This D-spectral feature is characteristic of the wurtzite-ZnO crystal structure, and it is very sensitive to small modifications of the local structure around Zn. As shown in Figure 8, the Zn K-edge XANES spectrum of the (ZnO4nm/ZnS4nm)10 heterostructure shows a profile very similar to that of bulk ZnO, in agreement with the high crystallinity and the formation of neat interfaces derived from X-Ray Reflectivity (XRR). In contrast, the spectrum of the Zn−O−S film prepared by copsuttering does not exhibit the characteristic spectral features of w-ZnS or w-ZnO systems but a rounded maximum indicating the amorphous character of the sample. Besides, while a nonzero XMCD signal is found in the case of the heterostructures, no detectable XMCD and thus no hint of saturation was found in the case of the sample made by cosputtering. These results confirm the need for pristine ZnS−ZnO interfaces to obtain FML behaviour in these systems. These results suggest that the higher crystallinity of the ZnO core would lead to the formation of a highly ordered interface between the ZnO core and the ZnS shell yielding an FML−ZnO contribution.
4. Conclusion
Through the course of this long-term study we have performed an extensive study of the ZnO NPs capped with different molecules and of ZnS/ZnO heterostructures by using different experimental techniques. The X-ray magnetic circular dichroism results demonstrated that the magnetism in these materials is intrinsic and relays in the ZnO conduction band. Moreover, both X-ray absorption spectroscopy (XAS) and XMCD signals pointed out the formation of a well defined interface between ZnO and the capping molecule in which the exotic magnetism arises at the hybridized band formed among Zn and the bonding atom of the molecule. Accordingly, the occurrence of magnetism should be related to the structural modification at the interface between the NPs and the molecules rather to the specific electronegativity of the atoms involved in the bonds at this interface.
The structural modifications occurring in ZnO nanoparticles when they are capped with organic molecules were studied by means of XANES at the Zn K-edge. In the case of thiol-capped samples, the comparison of the experimental spectra and ab-initio computations indicated the formation of a well defined ZnS interface at the surface of the nanoparticle in which ZnS adopts the local structure of wurtzite. These findings suggested that the exotic magnetism observed for these NPs is related to this interface, whose details should determine the particular magnetic properties of each system. In this way, it would be possible to reconcile previous contradictory reports on the magnetism of seemingly identical materials in terms of the formation of this interface. Further XAS and XMCD studies provide the demonstration that the modification of the surface of ZnO nanoparticles through the capping with organic molecules enables the development of ferromagnetic behaviour up to room temperature. The analysis of the XMCD spectra indicates the coexistence of both Pauli paramagnetism (hindered by diamagnetism to macroscopic magnetometry tools) and intrinsic ferromagnetism in the samples. The contribution of the PP to the XMCD stems form the wurtzite-like ZnS and ZnO ordered regions of the sample while ferromagnetism originates at the interface formed between the ZnS shell and the ZnO core. Zn K-edge XMCD vs. H measurements reveal, from the observed saturation at moderate applied fields, an intrinsic ferromagnetic-like contribution stemming from the formed interface that is estimated to extent over 3 to 8 Å depending the capping molecule. Our results also indicate that within this interface ferromagnetism is favoured in those regions of the interface where the local order is closer to w-ZnO than to w-ZnS.
All the results above were verified on different series of samples aimed of finally establishing the relationship between magnetic behaviour and local structure of ZnO nanoparticles capped with different organic molecules. The combined analysis performed by using different characterization tools, including atom-specific XAS and XMCD, demonstrates that the modification of the surface of ZnO nanoparticles through the capping with organic molecules enables the development of ferromagnetic behaviour up to room temperature. The results indicate that the occurrence of ferromagnetism does not critically depends on the details of the synthesis leading to different nanoparticle crystalline size or on the length of the organic molecule (butanethiol, octanethiol and dodecanethiol) but on the formation of a pristine ZnS-ZnO interface. The fact that all the samples show similar magnetic properties despite the different surface to bulk ratio indicates that ferromagnetism originates at this interface and not at the bulk-like components of the nanoparticles. Contrary to the commonly accepted view, the magnetism arise at the oxide conduction band, providing an avenue to explain the surprising results found in this field, sometimes seemingly irreconcilable. These results end the longstanding controversy about the existence of intrinsic RTFM in ZnO-based systems, providing new insights to finally establish the mechanism that sets on the ferromagnetic order in these systems and bringing support to this new route for room-temperature semiconductor spintronics.
References
[1] Chambers, S. A. Ferromagnetism in doped thin-film oxide and nitride semiconductors and dielectrics. Surf. Sci. Rep. 2006; 61: 345–381.
[2] Pearton, S. J.; Heo, W. H.; Ivill, M.; Norton, D. P.; Steiner, T. Semicond. Sci. Technol. 2004; 19, R59–R74.
[3] Sato, K.; Katayama-Yoshida, H. Ferromagnetism in a transition metal atom doped ZnO. Physica E 2001; 10: 251–255.
[4] Matsumoto, Y.; Murakami, M.; Shono, T.; Hasegawa, T.; Fukumura, T.; Kawasaki, M.; Ahmet, P.; Chikyow, T.; Koshihara, S.; Koinuma, H. Room-Temperature Ferromagnetism in Transparent Transition Metal-Doped Titanium Dioxide. Science 2001; 291: 854–856.
[5] Coey, J. M. D.; Chambers, S. A. Oxide Dilute Magnetic Semiconductors - Fact or Fiction? MRS Bulletin 2008; 33: 1053–1058.
[6] Kittilstved, K. R.; Liu, W. K.; Gamelin, D. R. Electronic structure origins of polarity-dependent high-TC ferromagnetism in oxide-diluted magnetic semiconductors. Nature Mater. 2006; 5: 291–297.
[7] Zunger, A.; Lany, S.; Raebiger, H. The quest for dilute ferromagnetism in semiconductors: Guides and misguides by theory. Physics 2010; 3: 53.
[8] Chambers, S. Is it really intrinsic ferromagnetism? Nat. Mater. 2010; 9: 956–957.
[9] Lawes, G.; Risbud, A. S.; Ramíırez, A. P.; Seshadri, R. Absence of ferromagnetism in Co and Mn substituted polycrystalline ZnO. Phys. Rev. B 2005; 71: 045201/1–045201/5.
[10] García, M. A.; Pinel, E. F.; de la Venta, J.; Quesada, A.; Bouzas, V.; Fernández, J. L.; Romero, J. L.; Martín-González, M. S.; Costa-Krämer, J. L. Sources of experimental errors in the observation of nanoscale magnetism. J. Appl. Phys. 2009; 105: 013925/1–7.
[11] Barla, A.; Schmerber, G.; Beaurepaire, E.; Dinia, A.; Bieber, H.; Colis, S.; Scheurer, F.; Kappler, J. P.; Imperia, P.; Nolting, F.; Wilhelm, F.; Rogalev, A.; Müller, D.; Grob, J. J. Paramagnetism of the Co sublattice in ferromagnetic Zn1-xCoxO films. Phys. Rev. B 2007; 76: 125201/1–5.
[12] Céspedes, E.; Laguna-Marco, M. A.; Jiménez-Villacorta, F.; Chaboy, J.; Boada, R.; Guglieri, C.; Andrés, A. de.; Prieto, C. On the Origin of the Magnetism of Mn-Zn-O Systems: Structural, Electronic, and Magnetic Study of Exotic MnO2-δ/ZnO Thin Films. The Journal of Physical Chemistry C 2011; 115: 24092–24101.
[13] Keavney, D. J.; Buchholz, D. B.; Ma, Q.; Chang, R. P. H. Where does the spin reside in ferromagnetic Cu-doped ZnO? Appl. Phys. Lett. 2007; 91: 012501/1–3.
[14] Coey, J. M. D. Dilute magnetic oxides. Current opinion in Solid State and. Mat. Sci. 2006; 10, 83–92.
[15] Carmeli, I.; Leitus, G.; Naaman, R.; Reich, S.; Vager, Z. Magnetism induced by the organization of self-assembled monolayers. J. Chem. Phys. 2003; 118: 10372–10375.
[16] Coey, J. M. D.; Venkatesan, M.; Fitzgerald, C. B. Donor impurity band exchange in dilute ferromagnetic oxides. Nature Mater. 2005; 4: 173–179.
[17] García, M. A.; Merino, J. M.; Fernández Pinel, E.; Quesada, A.; de la Venta, J.; Ruíz-González, M. L.; Castro, G. R.; Crespo, P.; Llopis, J.; González-Calbet, J. M.; Hernando, A. Magnetic Properties of ZnO Nanoparticles. Nano Letters 2007; 7: 1489–1494.
[18] T. Dietl, Dilute magnetic semiconductors: Functional ferromagnets. Nat. Mater. 2003; 2, 646-648.
[19] Chaboy, J.; Boada, R.; Piquer, C.; Laguna-Marco, M. A.; García-Hernández, M.; Carmona, N.; Llopis, J.; Ruíz-González, M. L.; González-Calbet, J.; Fernández, J. F.; García, M. A. Evidence of intrinsic magnetism in capped ZnO nanoparticles. Phys. Rev. B 2010; 82: 064411/1–9.
[20] Guglieri, C.; Laguna-Marco, M. A.; García, M. A.; Carmona, N.; Céspedes, E.; García-Hernández, M.; Espinosa, A.; Chaboy, J. XMCD proof of ferromagnetic behaviour in ZnO nanoparticles. J. Phys. Chem. C 2012; 116: 6608–6614.
[21] Guglieri, C.; Espinosa, A.; Carmona, N.; Laguna-Marco, M. A.; Céspedes, E.; Ruíz-González, M. L.; González-Calbet, M.; García-Hernández, M.; García, M. A.; Chaboy, J. Relationship between the magnetic properties and the formation of a ZnS/ZnO interface in S-capped ZnO nanoparticles and ZnS-ZnO thin films. J. Phys. Chem. C 2013; 117: 12199–12209.
[22] Guglieri, C.; Chaboy, J. Characterization of the ZnO-ZnS Interface in THIOL-Capped ZnO Nanoparticles Exhibiting Anomalous Magnetic Properties. J. Phys. Chem. C 2010; 114: 19629–19634.
[23] Hsu, H. S.; Huang, J. C. A.; Huang, Y. H.; Liao, Y. F.; Lin, M. Z.; Lee, C. H.; Lee, J. F.; Chen, S. F.; Lai, L. Y. and Liu, C. P. Evidence of oxygen vacancy enhanced room-temperature ferromagnetism in Co-doped ZnO. Appl. Phys. Lett. 2006; 88. 242507/1-3.
[24] Yan, W.; Sun, Z.; Liu, Q.; Li, Z.; Pan, Z.; Wang, J. and Wei, S. Zn vacancy induced room-temperature ferromagnetism in Mn-doped ZnO. Appl. Phys. Lett. 2007; 91: 062113/1-3.
[25] Zhang, S.; Zhang, L.; Li, H.; Jiang, Z.; Chu, W.; Huang, Y.; Wang, J. and Wu, Z. Investigation of annealing-induced oxygen vacancies in the Co-doped ZnO system by Co K-edge XANES spectroscopy. J. Synchrotron Radiat. 2010; 17: 600-605.
[26] Guglieri, C.; Céspedes, E.; Prieto, C.; Chaboy, J. X-ray absorption study of the local order around Mn in Mn:ZnO thin films: the role of vacancies and structural distortions. J. Phys. Condens. Matter 23 (2011) 206006 2011; 23: 206006/1-8.








