Volume 17, No.3 Pages 232 - 237
1. 最近の研究から/FROM LATEST RESEARCH
Long-term Proposal Report 2:
Phase Contrast X-ray Imaging of the Lung
[1]The Ritchie Centre, Monash Institute for Medical Research, [2]School of Physics, Monash University, [3]Division of Biological Engineering, Monash University, [4]Department of Radiography and Medical Imaging, Monash University, [5]Research & Utilization Division, JASRI
- Abstract
- Lung diseases have a major impact on human health at every stage of life. In newborn infants, respiratory failure is the greatest cause of death and disease, whereas in children, asthma and related airway diseases are a major cause of illness. Similarly, asthma, emphysema and pulmonary fibrosis are major causes of death in adults, but the diagnostic capabilities of current lung imaging modalities are limited by relatively poor spatial and temporal resolutions. Phase contrast (PC) X-ray imaging can image the lung with greatly increased temporal and spatial resolutions, compared with many other modalities. We have applied this technique to investigate factors that promote lung aeration following very premature birth and to measure alterations in lung motion caused by disease. We have demonstrated that lung aeration predominantly results from pressure gradients generated during inspiration, which has overturned almost 40 years of accepted wisdom. Because of this new understanding, we have developed new approaches for facilitating lung aeration in very premature newborns. Specifically we have shown that application of an end-expiratory pressure facilitates lung aeration by preventing liquid from re-entering the airways during expiration. We have also shown that a sustained inflation greatly promotes uniform lung aeration before tidal breathing begins and that expired CO2 levels accurately indicate the degree of lung aeration immediately after birth. By combining PC X-ray imaging with particle image velocimetry, we have also been able to identify how lung diseases affect the speed and direction of lung motion. This technique has the capability of measuring regional lung function with a spatial resolution that is unparalleled by any other current technology.
Introduction
Respiratory diseases are a major cause of death and disease in humans, which affects people at all stages of their lives. As the lungs are filled with liquid at birth, the onset of air-breathing is often impeded by the presence of liquid in the gas exchange regions of the lung which results in respiratory failure. In children, asthma is a common disease whereby airway constriction restricts gas flow through the airways, which can severely limit respiratory function resulting in death. Similarly, pulmonary fibrosis, emphysema and asthma are all major health issues in adults[1][1]Scott IA. Chronic disease management: a primer for physicians. Intern. Med. J. 2008; 38(6): 427-37.. Although our research has mainly focused on lung aeration and the transition to air-breathing at birth, the technologies we have developed can also be used to image adult lung diseases, such as pulmonary fibrosis and asthma.
During fetal life, the future airways of the lungs are filled with a liquid that must be cleared at birth to allow the entry of air and the onset of pulmonary gas exchange[2-4][2]Hooper SB, Harding R. Fetal lung liquid: A major determinant of the growth and functional development of the fetal lung. Clin. Exp. Pharmacol. Physiol. 1995; 22(4): 235-47.
[3]Olver RE, Walters DV, Wilson M. Developmental regulation of lung liquid transport. Annu. Rev. Physiol. 2004; 66: 77-101.
[4]Bland RD. Loss of liquid from the lung lumen in labor: more than a simple "squeeze". American Journal of Physiology-Lung Cellular and Molecular Physiology. 2001; 280(4): L602-L5.. This process of lung aeration triggers major physiological changes which are critical for the transition to life after birth[5, 6][5]Hooper SB, Harding R. Role of aeration in the physiological adaptation of the lung to air-breathing at birth. Curr. Resp. Med. Rev. 2005; 1: 185-95.
[6]Rudolph AM. Fetal and neonatal pulmonary circulation. Annu. Rev. Physiol. 1979; 41: 383-935.. These changes include a large increase in pulmonary blood flow (PBF) and closure of major vascular shunts that allow blood to by-pass the lungs during fetal life. Thus, the process of lung aeration underpins the transition to life after birth, not only by allowing the onset pulmonary gas exchange but by also initiating a cascade of related critical physiological events.
Until recently, airway liquid clearance was thought to result from Na+ reabsorption across the pulmonary epithelium which created an osmotic gradient for the movement of water from the airway lumen into the surrounding tissue[3][3]Olver RE, Walters DV, Wilson M. Developmental regulation of lung liquid transport. Annu. Rev. Physiol. 2004; 66: 77-101.. However, using phase contrast (PC) X-ray imaging, we have demonstrated that inspiration, rather Na+ reabsorption, was the primary force driving airway liquid clearance at birth[7, 8][7]Kitchen MJ, Lewis RA, Hooper SB, Wallace MJ, Siu KKW, Williams I, et al. Dynamic Studies of Lung Fluid Clearance with Phase Contrast Imaging. Ninth International Conference on Synchrotron Radiation Instrumentation; 2007 May 28 - June 2, 2006; Daegu, Korea: American Institute of Physics; 2007. p. 1903-7.
[8]Siew ML, te Pas AB, Wallace MJ, Kitchen MJ, Lewis RA, Fouras A, et al. Positive end-expiratory pressure enhances development of a functional residual capacity in preterm rabbits ventilated from birth. Journal of Applied Physiology. 2009; 106 (5): 1487-93..
Phase contrast X-ray imaging
PC X-ray imaging uses the phase changes produced by a X-ray beam as it propagates through an object that is comprised of material with differing refractive indices, such as air and water[9-11][9]Yagi N, Suzuki Y, Umetani K, Kohmura Y, Yamasaki K. Refraction-enhanced x-ray imaging of mouse lung using synchrotron radiation source. Medical Physics. 1999; 26(10): 2190-3.
[10]Kitchen MJ, Lewis RA, Yagi N, Uesugi K, Paganin D, Hooper SB, et al. Phase contrast x-ray imaging of mice and rabbit lungs: a comparative study. Br. J. Radiol. 2005; 78(935): 1018-27.
[11]Lewis RA, Yagi N, Kitchen MJ, Morgan MJ, Paganin D, Siu KKW, et al. Dynamic imaging of the lungs using X-ray phase contrast. Phys. Med. Biol. 2005; 50(21): 5031-40.. The phase changes produce interference patterns at a finite distance beyond the object, providing contrast of the boundaries between structures with differing refractive indices[11, 12][11]Lewis RA, Yagi N, Kitchen MJ, Morgan MJ, Paganin D, Siu KKW, et al. Dynamic imaging of the lungs using X-ray phase contrast. Phys. Med. Biol. 2005; 50(21): 5031-40.
[12]Kitchen MJ, Paganin D, Lewis RA, Yagi N, Uesugi K, Mudie ST. On the origin of speckle in x-ray phase contrast images of lung tissue. Physics in Medicine & Biology. 2004; 49(18): 4335-48.. When using coherent X-rays, the phase contrast between air and water are orders of magnitude larger than the change in absorption contrast produced by soft tissues[11][11]Lewis RA, Yagi N, Kitchen MJ, Morgan MJ, Paganin D, Siu KKW, et al. Dynamic imaging of the lungs using X-ray phase contrast. Phys. Med. Biol. 2005; 50(21): 5031-40.. The lung is ideally suited to this type of imaging[9-11][9]Yagi N, Suzuki Y, Umetani K, Kohmura Y, Yamasaki K. Refraction-enhanced x-ray imaging of mouse lung using synchrotron radiation source. Medical Physics. 1999; 26(10): 2190-3.
[10]Kitchen MJ, Lewis RA, Yagi N, Uesugi K, Paganin D, Hooper SB, et al. Phase contrast x-ray imaging of mice and rabbit lungs: a comparative study. Br. J. Radiol. 2005; 78(935): 1018-27.
[11]Lewis RA, Yagi N, Kitchen MJ, Morgan MJ, Paganin D, Siu KKW, et al. Dynamic imaging of the lungs using X-ray phase contrast. Phys. Med. Biol. 2005; 50(21): 5031-40. because it is predominantly comprised of air (~80% by volume at end expiration), surrounded by thin tissue structures (predominantly water). The air-tissue interfaces provide strong contrast, allowing the air-filled structures, which weakly absorb X-rays, to become visible[11, 12][11]Lewis RA, Yagi N, Kitchen MJ, Morgan MJ, Paganin D, Siu KKW, et al. Dynamic imaging of the lungs using X-ray phase contrast. Phys. Med. Biol. 2005; 50(21): 5031-40.
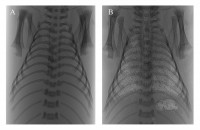
[12]Kitchen MJ, Paganin D, Lewis RA, Yagi N, Uesugi K, Mudie ST. On the origin of speckle in x-ray phase contrast images of lung tissue. Physics in Medicine & Biology. 2004; 49(18): 4335-48.. As the liquid-filled fetal lungs have no air/liquid interfaces they exhibit little phase or absorption contrast and so are not visible at birth, but progressively become visible as they aerate (Fig. 1). We have used BL20B2 at SPring-8, because of its high coherence and relative brightness, to image the entry of air into the lungs after birth and determine the primary factors involved.
Propagation-based phase contrast X-ray images of newborn rabbits during lung aeration acquired on BL20B2 at SPring-8 using 24 keV X-rays and an object-to-detector distance of 3 m. (A) Before the onset of air-breathing, the lungs are liquid-filled and not visible. (B) After numerous breaths the lungs are highly visible due to both phase and absorption contrast.
The PC X-ray images not only provide temporal and spatial information on the entry of air into the lungs at birth, but can also be used to measure regional lung air volumes and to track lung motion. We have developed algorithms that can measure air volume in different lung regions on a breath-by-breath basis allowing dynamic measures of gas distribution during ventilation[7, 13, 14][7]Kitchen MJ, Lewis RA, Hooper SB, Wallace MJ, Siu KKW, Williams I, et al. Dynamic Studies of Lung Fluid Clearance with Phase Contrast Imaging. Ninth International Conference on Synchrotron Radiation Instrumentation; 2007 May 28 - June 2, 2006; Daegu, Korea: American Institute of Physics; 2007. p. 1903-7.
[13]Kitchen MJ, Lewis RA, Morgan MJ, Wallace MJ, Siew ML, Siu KKW, et al. Dynamic measures of regional lung air volume using phase contrast x-ray imaging. Physics in medicine & biology. 2008; 53(21): 6065–77.
[14]Kitchen MJ, Paganin DM, Uesugi K, Allison BJ, Lewis RA, Hooper SB, et al. Phase contrast image segmentation using a Laue analyser crystal. Phys. Med. Biol. 2011; 56(3): 515-34.. We have also applied the technique of particle image velocimetry (PIV)[15][15]Fouras A, Dusting J, Lewis R, Hourigan K. Three-dimensional synchrotron x-ray particle image velocimetry. Journal of Applied Physics. 2007; 102(6): 064916. to the phase contrast X-ray image sequences, enabling us to characterize and measure regional lung motion[16][16]Fouras A, Kitchen MJ, Dubsky S, Lewis RA, Hooper SB, Hourigan K. The past, present and future of x-ray technology for in vivo imaging of function and form. J. Appl. Phys. 2009; 105(1): 102009-1-14. (see below).
Lung aeration at birth
Using PC X-ray imaging, we have determined the rate and spatial pattern of lung aeration from birth in newborn rabbits and examined the dynamics of the air/ liquid interface as it moves into the distal gas exchange regions of the lung[7, 13][7]Kitchen MJ, Lewis RA, Hooper SB, Wallace MJ, Siu KKW, Williams I, et al. Dynamic Studies of Lung Fluid Clearance with Phase Contrast Imaging. Ninth International Conference on Synchrotron Radiation Instrumentation; 2007 May 28 - June 2, 2006; Daegu, Korea: American Institute of Physics; 2007. p. 1903-7.
[13]Kitchen MJ, Lewis RA, Morgan MJ, Wallace MJ, Siew ML, Siu KKW, et al. Dynamic measures of regional lung air volume using phase contrast x-ray imaging. Physics in medicine & biology. 2008; 53(21): 6065–77.. Sequentially acquired images were used to construct videos that detailed the movement of air through the airways and into the distal gas exchange regions during the first breaths after birth. We found that the distal movement of air occurred rapidly and only during inspiration, whereas the air/liquid boundary commonly moved proximally during expiration and at rest; the latter most probably indicates liquid re-entry into the airways during expiration. As only 3-5 breaths were required to aerate the lungs, depending upon the size of the inspiratory efforts[17][17]Hooper SB, Kitchen MJ, Siew MLL, Lewis RA, Fouras A, te Pas AB, et al. Imaging lung aeration and lung liquid clearance at birth using phase-contrast x-ray imaging. Clinical and Experimental Pharmacology and Physiology. 2009; 36(1): 117-25., we concluded that the large trans-pulmonary hydrostatic pressures generated by inspiration provided the necessary pressure gradients for the movement of liquid from the lung lumen and into the surrounding tissue[18, 19][18]Hooper SB, Kitchen MJ, Wallace MJ, Yagi N, Uesugi K, Morgan MJ, et al. Imaging lung aeration and lung liquid clearance at birth. The Journal of the Federation of American Societies for Experimental Biology. 2007; 21(12): 3329-37.
[19]Siew ML, Wallace MJ, Kitchen MJ, Lewis RA, Fouras A, te Pas AB, et al. Inspiration regulates the rate and temporal pattern of lung liquid clearance and lung aeration at birth. Journal of Applied Physiology. 2009; 106(6): 1888-95.. The liquid is then cleared via the lymphatics and blood vessels[8][8]Siew ML, te Pas AB, Wallace MJ, Kitchen MJ, Lewis RA, Fouras A, et al. Positive end-expiratory pressure enhances development of a functional residual capacity in preterm rabbits ventilated from birth. Journal of Applied Physiology. 2009; 106 (5): 1487-93.. These findings have completely altered our understanding of the mechanisms driving airway liquid clearance at birth and have led to new approaches to facilitate this process in very premature infants[8, 17][8]Siew ML, te Pas AB, Wallace MJ, Kitchen MJ, Lewis RA, Fouras A, et al. Positive end-expiratory pressure enhances development of a functional residual capacity in preterm rabbits ventilated from birth. Journal of Applied Physiology. 2009; 106 (5): 1487-93.
[17]Hooper SB, Kitchen MJ, Siew MLL, Lewis RA, Fouras A, te Pas AB, et al. Imaging lung aeration and lung liquid clearance at birth using phase-contrast x-ray imaging. Clinical and Experimental Pharmacology and Physiology. 2009; 36(1): 117-25..
Improving lung aeration following premature birth
Infants born very premature (24-28 weeks gestation) have structurally immature lungs and have difficulty in clearing their airways of liquid largely because they have weak inspiratory muscles[20][20]Jobe AH, Hillman N, Polglase G, Kramer BW, Kallapur S, Pillow J. Injury and inflammation from resuscitation of the preterm infant. Neonatology. 2008; 94(3): 190-6.. As a result, these infants usually suffer respiratory failure at birth and require respiratory support to survive. Airway liquid retention is a major problem in very premature infants as it reduces the gas volume of the lung, restricts gas exchange and causes marked regional differences in gas distribution, which greatly increases the risk of injury[20][20]Jobe AH, Hillman N, Polglase G, Kramer BW, Kallapur S, Pillow J. Injury and inflammation from resuscitation of the preterm infant. Neonatology. 2008; 94(3): 190-6.. Thus, airway liquid clearance at birth is a critical issue in very preterm infants and the development of procedures that facilitate this process are of the highest priority[21][21]te Pas AB, Davis PG, Hooper SB, Morley CJ. From Liquid to Air: Breathing after Birth. The Journal of Pediatrics. 2008; 152(5): 607-11..
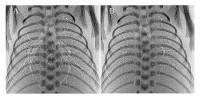
Based on our finding that trans-pulmonary pressures are the primary driving force for airway liquid clearance after birth, we have subsequently shown that ventilatory procedures that maintain the transpulmonary pressure gradient greatly facilitate uniform lung aeration in very preterm rabbits. For instance, a positive end-expiratory pressure (PEEP) applies a positive pressure to the airways throughout expiration, rather than allowing airway pressure to decrease to atmospheric pressure. In the absence of PEEP, the lungs failed to accumulate end-expiratory gas volumes (functional respiratory capacity; FRC) and either collapsed or re-filled with liquid during expiration (Fig. 2)[8, 22][8]Siew ML, te Pas AB, Wallace MJ, Kitchen MJ, Lewis RA, Fouras A, et al. Positive end-expiratory pressure enhances development of a functional residual capacity in preterm rabbits ventilated from birth. Journal of Applied Physiology. 2009; 106 (5): 1487-93.
[22]te Pas AB, Siew M, Wallace MJ, Kitchen MJ, Fouras A, Lewis RA, et al. Establishing Functional Residual Capacity at Birth: The Effect of Sustained Inflation and Positive End-Expiratory Pressure in a Preterm Rabbit Model. Pediatric Research. 2009; 65(5): 537-41.. Thus, PEEP greatly facilitated airway liquid clearance and, most importantly, allowed gas to remain within the gas exchange regions at end-expiration so that gas exchange could proceed throughout the respiratory cycle. Without PEEP, the gas exchange regions re-filled with liquid during expiration, thereby restricting gas exchange to the brief period of inspiration (30-50% of the respiratory cycle)[22, 23][22]te Pas AB, Siew M, Wallace MJ, Kitchen MJ, Fouras A, Lewis RA, et al. Establishing Functional Residual Capacity at Birth: The Effect of Sustained Inflation and Positive End-Expiratory Pressure in a Preterm Rabbit Model. Pediatric Research. 2009; 65(5): 537-41.
[23]te Pas AB, Siew M, Wallace MJ, Kitchen MJ, Fouras A, Lewis RA, et al. Effect of Sustained Inflation Length on Establishing Functional Residual Capacity at Birth in Ventilated Premature Rabbits. Pediatric Research. 2009; 66(3): 295-300..
Similarly, a sustained inflation (SI) not only applies a prolonged pressure to the airways to force liquid movement into lung tissue, but also increases the time for the pressure to overcome the high resistance to moving liquid (compared with air) through the airways[22, 23][22]te Pas AB, Siew M, Wallace MJ, Kitchen MJ, Fouras A, Lewis RA, et al. Establishing Functional Residual Capacity at Birth: The Effect of Sustained Inflation and Positive End-Expiratory Pressure in a Preterm Rabbit Model. Pediatric Research. 2009; 65(5): 537-41.
[23]te Pas AB, Siew M, Wallace MJ, Kitchen MJ, Fouras A, Lewis RA, et al. Effect of Sustained Inflation Length on Establishing Functional Residual Capacity at Birth in Ventilated Premature Rabbits. Pediatric Research. 2009; 66(3): 295-300.. Using PC X-ray imaging, we found that a 20 sec SI can completely aerate the lung from the first breath, resulting in full recruitment of FRC and tidal volume as well as uniform ventilation from the first breath. The combination of a SI and PEEP was found to be the best approach, as PEEP maintained FRC during subsequent tidal ventilation[22, 23][22]te Pas AB, Siew M, Wallace MJ, Kitchen MJ, Fouras A, Lewis RA, et al. Establishing Functional Residual Capacity at Birth: The Effect of Sustained Inflation and Positive End-Expiratory Pressure in a Preterm Rabbit Model. Pediatric Research. 2009; 65(5): 537-41.
[23]te Pas AB, Siew M, Wallace MJ, Kitchen MJ, Fouras A, Lewis RA, et al. Effect of Sustained Inflation Length on Establishing Functional Residual Capacity at Birth in Ventilated Premature Rabbits. Pediatric Research. 2009; 66(3): 295-300..
More recently, we have used our imaging approach to improve the feedback information that is used to guide clinical care in the delivery room. Currently, the gold standard of care uses transcutaneous oxygen saturation, heart rate and respiratory function monitoring in the delivery room[24-27][24]Dawson JA, Kamlin CO, Vento M, Wong C, Cole TJ, Donath SM, et al. Defining the reference range for oxygen saturation for infants after birth. Pediatrics. 2010; 125(6): e1340-7.
[25]Dawson JA, Kamlin CO, Wong C, te Pas AB, Vento M, Cole TJ, et al. Changes in heart rate in the first minutes after birth. Arch. Dis. Child. Fetal Neonatal Ed. 2010; 95(3): F177-81.
[26]Schmolzer GM, Kamlin OC, Dawson JA, te Pas AB, Morley CJ, Davis PG. Respiratory monitoring of neonatal resuscitation. Arch. Dis. Child. Fetal Neonatal Ed. 2010; 95(4): F295-303.
[27]Schmolzer GM, Morley CJ, Wong C, Dawson JA, Kamlin CO, Donath SM, et al. Respiratory function monitor guidance of mask ventilation in the delivery room: a feasibility study. J. Pediatr. 2012; 160(3): 377-81 e2.. However, these parameters provide little information on ventilation efficiency and the degree of pulmonary gas exchange and limited feedback to guide clinical care when cardiorespiratory indicators fail to improve. We have investigated whether expired CO2 levels can indicate when gas exchange first commences and the degree of aeration within the distal regions of the lung. This novel concept is based on the idea that CO2 exchange can only occur, and CO2 appear in the expired gas, only when the distal gas exchange regions of the lung aerate. Using simultaneous PC X-ray imaging and monitoring of expired CO2 levels we have demonstrated that CO2 appears in the expired air when only ~7% of the distal gas exchange structures have aerated and that the amount of expired CO2 directly correlates with gas volumes in the lung at end-inspiration of the preceding breath.
Matching ventilation with the increase in pulmonary blood flow at birth
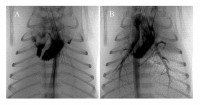
Before birth, pulmonary vascular resistance (PVR) is high and so blood flow through the lungs is low. Instead, most blood exiting the right ventricle by-passes the lungs and passes through the ductus arteriosis which connects the main pulmonary artery to the aorta. However, at birth, aeration of the lung decreases PVR, which increases pulmonary blood flow (PBF) and causes the ductus aretiosus to close so that all blood from the right ventricle passes through the lungs. The mechanism by which lung aeration decreases PVR is not clear, although increased oxygenation, the release of vasodilators and increased lung recoil caused by the creation of surface tension within the lung are all thought to be involved. We have recently combined PC X-ray imaging with angiography to examine the interaction between lung aeration and the resulting increase in PBF. To our surprise we found that the two were not spatially related and that regional lung aeration caused a global increase in PBF (Fig. 3). The mechanisms are unknown, but this represents and ideal model for examining and identifying them.
Tracking and measuring lung motion
As lung diseases principally restrict the ability of the lung to expand and deflate during breathing, we have investigated whether changes in lung motion caused by disease can be used to detect and measure the severity of disease. Pulmonary fibrosis increases the stiffness of lung tissue thereby reducing its capacity to expand, asthma increases airway resistance and emphysema reduces lung tissue recoil thereby increasing its compliance. Although lung motion must be altered in diseased regions, it is not known how different regions of the lung move in relation to other regions during both inspiration and expiration in a healthy lung. Similarly, it is unknown how diseases affect regional lung motion and whether motion in healthy regions is altered to compensate for diseased regions.
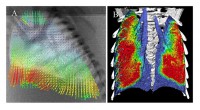
To investigate this, we have combined the high spatial and temporal resolution provided by PC X-ray imaging, with Particle Image Velocimetry (PIV) to measure regional lung motion[16][16]Fouras A, Kitchen MJ, Dubsky S, Lewis RA, Hooper SB, Hourigan K. The past, present and future of x-ray technology for in vivo imaging of function and form. J. Appl. Phys. 2009; 105(1): 102009-1-14.. PIV utilises a cross-correlation analysis to track the movement of small regions of lung tissue between consecutive frames. This allows reconstruction of velocity fields that define the speed and direction of regional lung motion throughout a breath (Fig. 4). These demonstrate that motion is very heterogeneous across the lung and that the speed and direction of motion within a region is highly position dependent; regions closest to the diaphragm move the greatest. Furthermore, we found that non-uniform lung disease (bleomycin-induced pulmonary fibrosis), caused abnormal motion in both diseased and healthy regions, with motion in healthy regions increasing to compensate for slower moving diseased regions[28][28]Fouras A, Allison BJ, Kitchen MJ, Dubsky S, Nguyen J, Hourigan K, et al. Altered Lung Motion: A sensitive indicator of regional lung disease. Submitted..
Phase contrast X-ray image of a newborn rabbit lung overlaid with velocity vectors (measured using PIV) showing regional lung motion at mid-inspiration, vector size and colour depicts the velocity magnitude (from lowest in blue to largest in red).
Further analysis of the velocity fields allows the derivation of expansion maps (Fig. 4) of the lung and, when analyzed in 3D using a computed tomography approach, also allows the calculation of gas flows in individual airways. Using this approach, we have demonstrated that bronchoconstriction, as occurs in asthma, causes major changes in lung expansion (Fig. 4B) and gas flow within individual airways. Future experiments are planned to determine the efficacy of action of inhaled bronchodilators.
Conclusion
PC X-ray imaging is an ideal technique for studying the factors that influence lung aeration and the clearance of airway liquid at birth as well as the impact that lung diseases have on dynamic lung function. Using this technology, we have identified ventilation strategies that most effectively aerate the lung after birth and promote uniform distribution of ventilation within the lung in very premature infants. The latter will likely reduce the lung injury suffered by premature infants requiring respiratory support. Our findings have prompted changes to guidelines recommending ventilation procedures that should be adopted in the delivery room immediately after birth in very immature infants (see Victorian Government, Australia website at: http://www.neoresus.org.au/pages/LM1-7-Breathing.php#B_FirstBreaths). Furthermore, PC- X-ray imaging provides sufficient spatial and temporal resolution that enables exploitation of analytical techniques such as X-ray PIV to quantify regional lung motion and detect lung disease by identifying regions with abnormal movement. If this technology can be adapted to X-ray sources available for clinical use, it promises to be a sensitive and quantitative method for detecting and monitoring lung disease.
References
[1]Scott IA. Chronic disease management: a primer for physicians. Intern. Med. J. 2008; 38(6): 427-37.
[2]Hooper SB, Harding R. Fetal lung liquid: A major determinant of the growth and functional development of the fetal lung. Clin. Exp. Pharmacol. Physiol. 1995; 22(4): 235-47.
[3]Olver RE, Walters DV, Wilson M. Developmental regulation of lung liquid transport. Annu. Rev. Physiol. 2004; 66: 77-101.
[4]Bland RD. Loss of liquid from the lung lumen in labor: more than a simple "squeeze". American Journal of Physiology-Lung Cellular and Molecular Physiology. 2001; 280(4): L602-L5.
[5]Hooper SB, Harding R. Role of aeration in the physiological adaptation of the lung to air-breathing at birth. Curr. Resp. Med. Rev. 2005; 1: 185-95.
[6]Rudolph AM. Fetal and neonatal pulmonary circulation. Annu. Rev. Physiol. 1979; 41: 383-935.
[7]Kitchen MJ, Lewis RA, Hooper SB, Wallace MJ, Siu KKW, Williams I, et al. Dynamic Studies of Lung Fluid Clearance with Phase Contrast Imaging. Ninth International Conference on Synchrotron Radiation Instrumentation; 2007 May 28 - June 2, 2006; Daegu, Korea: American Institute of Physics; 2007. p. 1903-7.
[8]Siew ML, te Pas AB, Wallace MJ, Kitchen MJ, Lewis RA, Fouras A, et al. Positive end-expiratory pressure enhances development of a functional residual capacity in preterm rabbits ventilated from birth. Journal of Applied Physiology. 2009; 106 (5): 1487-93.
[9]Yagi N, Suzuki Y, Umetani K, Kohmura Y, Yamasaki K. Refraction-enhanced x-ray imaging of mouse lung using synchrotron radiation source. Medical Physics. 1999; 26(10): 2190-3.
[10]Kitchen MJ, Lewis RA, Yagi N, Uesugi K, Paganin D, Hooper SB, et al. Phase contrast x-ray imaging of mice and rabbit lungs: a comparative study. Br. J. Radiol. 2005; 78(935): 1018-27.
[11]Lewis RA, Yagi N, Kitchen MJ, Morgan MJ, Paganin D, Siu KKW, et al. Dynamic imaging of the lungs using X-ray phase contrast. Phys. Med. Biol. 2005; 50(21): 5031-40.
[12]Kitchen MJ, Paganin D, Lewis RA, Yagi N, Uesugi K, Mudie ST. On the origin of speckle in x-ray phase contrast images of lung tissue. Physics in Medicine & Biology. 2004; 49(18): 4335-48.
[13]Kitchen MJ, Lewis RA, Morgan MJ, Wallace MJ, Siew ML, Siu KKW, et al. Dynamic measures of regional lung air volume using phase contrast x-ray imaging. Physics in medicine & biology. 2008; 53(21): 6065–77.
[14]Kitchen MJ, Paganin DM, Uesugi K, Allison BJ, Lewis RA, Hooper SB, et al. Phase contrast image segmentation using a Laue analyser crystal. Phys. Med. Biol. 2011; 56(3): 515-34.
[15]Fouras A, Dusting J, Lewis R, Hourigan K. Three-dimensional synchrotron x-ray particle image velocimetry. Journal of Applied Physics. 2007; 102(6): 064916.
[16]Fouras A, Kitchen MJ, Dubsky S, Lewis RA, Hooper SB, Hourigan K. The past, present and future of x-ray technology for in vivo imaging of function and form. J. Appl. Phys. 2009; 105(1): 102009-1-14.
[17]Hooper SB, Kitchen MJ, Siew MLL, Lewis RA, Fouras A, te Pas AB, et al. Imaging lung aeration and lung liquid clearance at birth using phase-contrast x-ray imaging. Clinical and Experimental Pharmacology and Physiology. 2009; 36(1): 117-25.
[18]Hooper SB, Kitchen MJ, Wallace MJ, Yagi N, Uesugi K, Morgan MJ, et al. Imaging lung aeration and lung liquid clearance at birth. The Journal of the Federation of American Societies for Experimental Biology. 2007; 21(12): 3329-37.
[19]Siew ML, Wallace MJ, Kitchen MJ, Lewis RA, Fouras A, te Pas AB, et al. Inspiration regulates the rate and temporal pattern of lung liquid clearance and lung aeration at birth. Journal of Applied Physiology. 2009; 106(6): 1888-95.
[20]Jobe AH, Hillman N, Polglase G, Kramer BW, Kallapur S, Pillow J. Injury and inflammation from resuscitation of the preterm infant. Neonatology. 2008; 94(3): 190-6.
[21]te Pas AB, Davis PG, Hooper SB, Morley CJ. From Liquid to Air: Breathing after Birth. The Journal of Pediatrics. 2008; 152(5): 607-11.
[22]te Pas AB, Siew M, Wallace MJ, Kitchen MJ, Fouras A, Lewis RA, et al. Establishing Functional Residual Capacity at Birth: The Effect of Sustained Inflation and Positive End-Expiratory Pressure in a Preterm Rabbit Model. Pediatric Research. 2009; 65(5): 537-41.
[23]te Pas AB, Siew M, Wallace MJ, Kitchen MJ, Fouras A, Lewis RA, et al. Effect of Sustained Inflation Length on Establishing Functional Residual Capacity at Birth in Ventilated Premature Rabbits. Pediatric Research. 2009; 66(3): 295-300.
[24]Dawson JA, Kamlin CO, Vento M, Wong C, Cole TJ, Donath SM, et al. Defining the reference range for oxygen saturation for infants after birth. Pediatrics. 2010; 125(6): e1340-7.
[25]Dawson JA, Kamlin CO, Wong C, te Pas AB, Vento M, Cole TJ, et al. Changes in heart rate in the first minutes after birth. Arch. Dis. Child. Fetal Neonatal Ed. 2010; 95(3): F177-81.
[26]Schmolzer GM, Kamlin OC, Dawson JA, te Pas AB, Morley CJ, Davis PG. Respiratory monitoring of neonatal resuscitation. Arch. Dis. Child. Fetal Neonatal Ed. 2010; 95(4): F295-303.
[27]Schmolzer GM, Morley CJ, Wong C, Dawson JA, Kamlin CO, Donath SM, et al. Respiratory function monitor guidance of mask ventilation in the delivery room: a feasibility study. J. Pediatr. 2012; 160(3): 377-81 e2.
[28]Fouras A, Allison BJ, Kitchen MJ, Dubsky S, Nguyen J, Hourigan K, et al. Altered Lung Motion: A sensitive indicator of regional lung disease. Submitted.