Volume 25, No.4 Pages 284 - 288
1. 最近の研究から/FROM LATEST RESEARCH
(SPRUC 2020 Young Scientist Award Winner Research Report)
Development of X-ray Transparent Cell in Large Volume Press towards Silicate Melt Viscosity Measurement under Lower-Mantle Conditions
Institute for Planetary Materials, Okayama University / Bayerisches Geoinstitut, University of Bayreuth
- Abstract
- High-pressure and high-temperature experiments are indispensable to understand the Earth's interior. Large volume press (LVP) can generate pressures in a much larger volume than that of the diamond anvil cell, and has been widely used in various X-ray measurements revealing physical and chemical properties of minerals under the Earth's mantle conditions. Development of X-ray transparent cell in LVP is, thus, essential. In this study, I developed a boron-doped diamond (BDD) heating element, which is both refractory and X-ray transparent; and a boron-MgO composite pressure medium, which is highly X-ray transparent without sacrificing the pressure generation efficiency. With BDD heater, I succeeded in generating temperature as high as 4000 K at ~15 GPa. Then, I tried to apply the BDD heating element to in-situ falling sphere viscometry, which is the best method to directly measure viscosity under high-pressure conditions. Thanks to the ideal characteristics of BDD heater, I succeeded in measuring viscosity of forsterite, enstatite and diopside liquids up to 30 GPa by in-situ falling sphere viscometry. The new viscosity data set infers that a bridgmanite-enriched layer should form at the top lower-mantle during the cooling of magma ocean.
I. Introduction
The Earth's interior is under high pressure (to 136 GPa in the mantle, to 360 GPa in the core) and high temperature (to ~4000 K in the mantle, to ~5500 K in the core). The physical and chemical properties of Earth material under high-pressure and high-temperature (HPHT) is crucial to understand the Earth's interior[1][1] E. Ito: Miner. Phys. 2 (2007) 197-230. (doi: 10.1016/b978-044452748-6/00036-5). HPHT experiment is an indispensable tool to reveal how materials behave in the Earth's interior. Diamond anvil cell and large volume press (LVP) are two complementary apparatuses to generate HPHT conditions. Comparing them, LVP (Fig.1a) can generate pressures in a much larger volume with a fine temperature control and reduced thermal gradients[1][1] E. Ito: Miner. Phys. 2 (2007) 197-230. (doi: 10.1016/b978-044452748-6/00036-5).
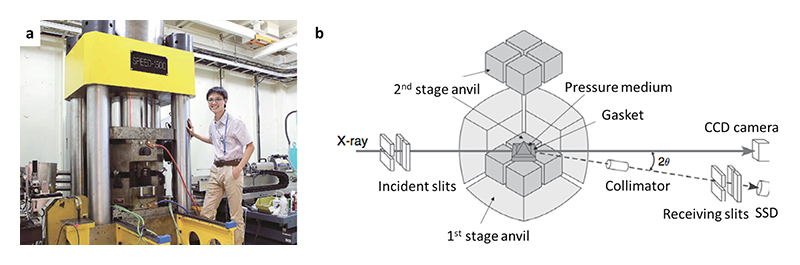
Figure 1 (a) Dr. Xie doing experiment with an LVP installed at BL04B1. (b) X-ray optics of an LVP interfaced with synchrotron X-ray (modified from Ito, 2007[1][1] E. Ito: Miner. Phys. 2 (2007) 197-230. (doi: 10.1016/b978-044452748-6/00036-5)).
Synchrotron X-ray is an indispensable tool to reveal physical and chemical properties of materials. The combination of LVP and synchrotron X-ray recently expanded the versatility of LVP greatly, with studies ranging from phase diagram and equation of state by X-ray diffraction[2 and reference therein][2] T. Katsura: Spec. Pap. Geol. Soc. Am. 421 (2007) 189-205., to viscosity measurement by X-ray radiography[e.g.3][3] M. Kanzaki et al.: High-Pressure Research in Mineral Physics: A 39 (2011) 195-200. (doi:10.1029/gm039p0195), and density determination in liquids by X-ray absorption[e.g.4][4] T. Sakamaki, E. Ohtani, S. Urakawa, A. Suzuki and Y. Katayama: Earth Planet. Sci. Lett. 287 (2009) 293-297.. Fig.1b shows a schematic drawing of X-ray optics in an LVP. Since the X-ray penetrates the cell assembly, X-ray transparent material making up the cell is required for high signal/noise ratio.
II. Development of X-ray transparent material
A. Boron-doped diamond heater
LVP usually uses resistive heating to generate high temperatures. X-ray transparency of heating element is, therefore, essential for X-ray measurements at HPHT. Graphite is an ideal heating element in X-ray transparent cell below 10 GPa because of its high melting point (~5000 K) and high X-ray transparency. Unfortunately, it converts to diamond at higher pressures. Since diamond is an electrical insulator, graphite cannot be used as a heater at diamond stable P-T ranges. LaCrO3 or noble metals are used instead. However, both of them are X-ray opaque and not refractory enough (< 3300 K). X-ray transparent and refractory heating element for pressures > 10 GPa has long been awaited.
Even though pure diamond is electrical insulator, it becomes semi-conductive or even metallic when heavily doped with boron. Furthermore, diamond is refractory with a melting point of ~5000 K. Therefore, boron-doped diamond (BDD) is the best candidate as a refractory and X-ray transparent heating element in LVP at diamond stable P-T conditions. The pioneer researches were focused on the direction of using a graphite–boron mixture as precursor, which converts to BDD in situ during heating, to avoid direct manufacturing the hardest material, diamond. However, a significant pressure drop and unstable heating are frequently caused by the graphite-diamond conversion, which is accompanied by a large volume reduction and drastic change of electrical resistivity[5-8][5] A. Yamada et al.: High Press. Res. 28 (2008) 255-264.
[6] A. Shatskiy et al.: Rev. Sci. Instrum. 80 (2009) 023907.
[7] A. Yoneda, L. Xie, N. Tsujino and E. Ito: High Press. Res. 34 (2014) 392-403.
[8] L. Xie, A. Yoneda, T. Yoshino, H. Fei and E. Ito: High Press. Res. 36 (2016) 105-120.. In addition, boron in the mixture may be oxidized into boron oxide, B2O3, which acts as a fatal melting flux[8][8] L. Xie, A. Yoneda, T. Yoshino, H. Fei and E. Ito: High Press. Res. 36 (2016) 105-120..
Under this context, I turned to use pre-synthesized BDD as a starting material instead of graphite-boron mixture. To overcome the difficulty of manufacturing BDD tube, I directly synthesized BDD tubes at HPHT or molded a BDD tube from BDD powders, which were crashed from BDD blocks using a nano-polycrystalline diamond mortar. Fig.2a shows images of BDD blocks and tubes. After surveying various refractory materials, I found that TiC is the best electrode for BDD heater. Owing to these breakthroughs, I succeeded to generate ~4000 K in LVP; it is higher than the geotherm of the whole Earth's mantle and over the melting temperature of Earth's mantle to 2400 km, close to the core mantle boundary (see Fig.2b). Being both refractory and X-ray transparent, BDD shows great advantage than any other type of furnaces for synchrotron X-ray studies and opens new opportunities to perform unprecedented experiments, such as exploring the silicate melt properties at Earth's lower mantle conditions. This work has been published in Review of Scientific Instrument, 2017[9][9] L. Xie et al.: Rev. Sci. Instrum. 88 (2017) 093904..
It is worth noting that not only LVP researchers are interested in the BDD material, but also diamond anvil cell researchers collaborate with me to generate 3500 K in diamond anvil cell for 1 hour duration[10][10] H. Ozawa et al.: High Press. Res. 38 (2018) 120-135..
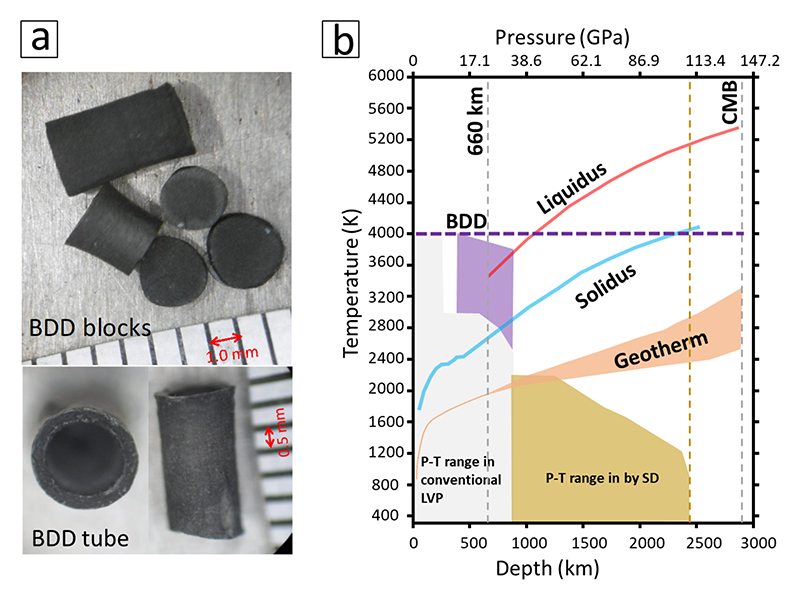
Figure 2 (a) Image of BDD blocks and tubes, synthesized at 15 GPa and 2300 K. (b) Available pressure-temperature range in LVP, which is modified after Yamazaki et al. 2014. CMB: core-mantle boundary. Purple dashed line marks the temperature of 4000 K, the highest temperature generated by BDD heater. Purple area marks the pressure-temperature (P-T) range, in which BDD was applied to measure melt viscosity[15][15] L. Xie et al.: Nat. Commun. 11 (2020) 548.; grey area marks that in conventional LVP, which uses WC as second stage anvils; yellow area marks that in LVP, which uses sintered diamond (SD) as second stage anvils.
B. Boron-MgO composite pressure medium
Pressure medium (PM) is a material which is used to transmit pressure from the anvil to the sample. Besides heating element, X-ray transparent PM is important as well in enhancing transparency of cell assembly. A good PM for in-situ X-ray measurements should be X-ray transparent without scarifying efficiency of pressure generation. Traditional PM has limited X-ray transparency such as Cr-doped MgO (~5 wt.% Cr2O3) or limited pressure generation such as B/Epoxy mixture (practically < 10 GPa).
For these reasons, I developed B85 (85 wt.% B and 15 wt.% Mg(OH)2) as PM. I sintered machinable blocks of boron-MgO composite at 800-1000°C under atmospheric pressure from a mixture of amorphous boron and brucite or Mg(OH)2. Fig.3 shows various shapes of sintered boron-MgO composite blocks. B85 PM has significantly higher X-ray transparency, especially at energies less than 50 keV (Fig.4b). I also confirmed feasibility of the B85 PM by successfully generating lower-mantle pressure (> 23 GPa) with an efficiency comparable to that of a Cr-doped MgO PM (Fig.4c). In short, B85 is not only X-ray transparent but also efficient in pressure generation. Therefore, B85 PM enables us to conduct various cutting-edge X-ray measurements to lower mantle conditions in LVP, such as melt-structure measurement by the X-ray scattering method and density measurement using X-ray absorption method. The superiority of B85 PM was reported in Review of Scientific Instruments (2020) with citation of Editor's Pick[11][11] L. Xie et al.: Rev. Sci. Instrum. 91 (2020) 043903..
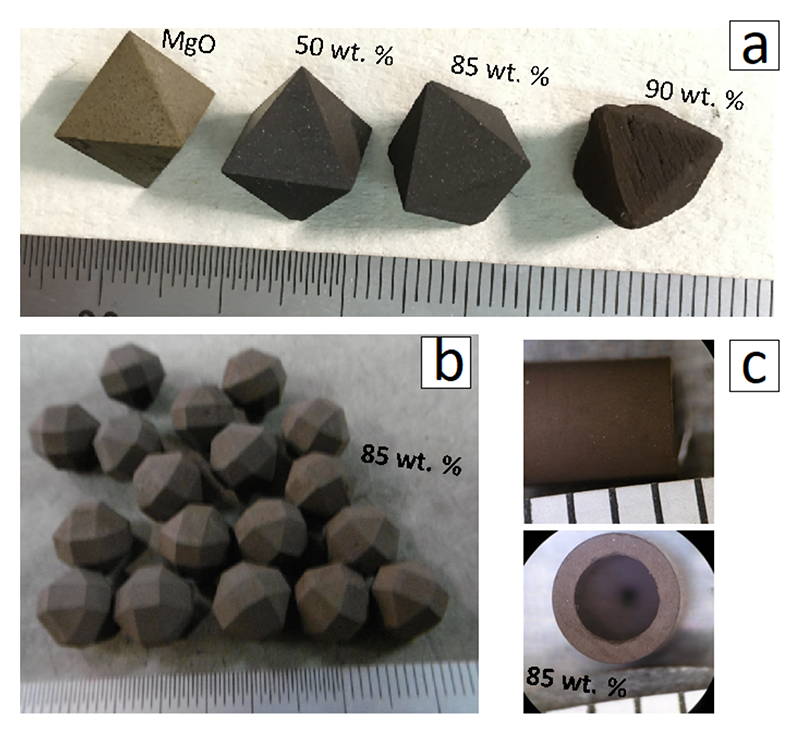
Figure 3 Varies shapes of boron-MgO composite. (a) Octahedra (10 mm edge length) machined from blocks with different boron contents. (b) Edges and vertexes truncated octahedra machined from B85. (c) Tube with OD/ID/L of 3.0/2.0/4.0 mm (OD: outer diameter, ID: inner diameter, L: length) machined from B85.
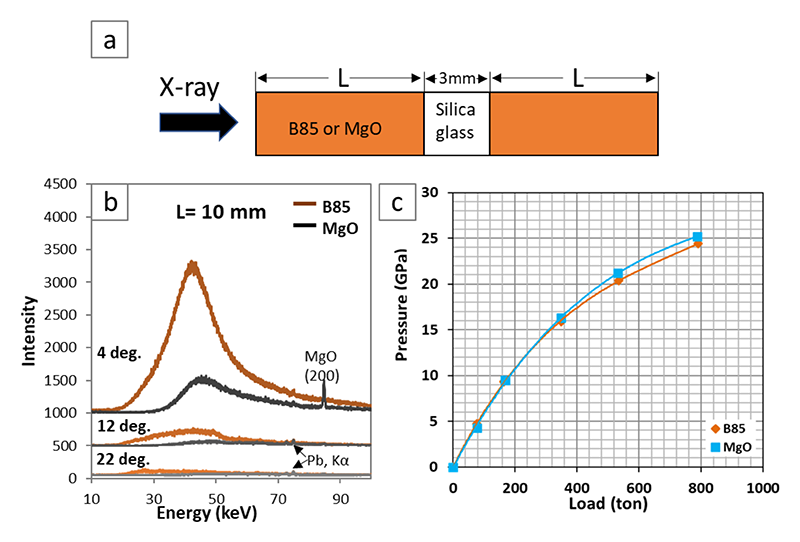
Figure 4 (a) Schematic sketch of X-ray passage to test X-ray transparency of pressure medium. B85: boron-MgO composite with 85 wt.% boron. X-ray beam was of 0.1 × 0.2 mm width. (b) X-ray diffraction spectra of silica glass at varying 2theta angle. Spectra were obtained at SPring-8 with an exposure time of 600 s. (c) Load-pressure relation using pressure medium of B85 and MgO, respectively, conducted at synchrotron SOLEIL (France). The experiments were performed using octahedron pressure medium with 10 mm edge length and tungsten carbide cubic anvils with 4 mm truncated edge length.
III. Application of BDD to viscosity measurement of silicate melts under lower-mantle conditions
The early Earth is believed to experience large-scale melting, which forms a deep or even whole-mantle magma ocean (MO)[12][12] W. B. Tonks and H. J. Melosh: J. Geophys. Res., Planets 98 (1993) 5319-5333.. Depending on the dominant mechanism of MO solidification (at equilibrium or with compositional fractionation), the primordial Earth's mantle could be layer-stratified or homogeneous. Besides the heat flux (a flow of energy per unit of area per unit of time) of MO surface, viscosity is a key parameter to characterize the solidification process[13][13] V. Solomatov: Treatise Geophys. Second Ed. 9 (2015) 81-104..
The viscosity of a fluid is a measure of its resistance to deformation at a given rate. For example, honey has higher viscosity than water. In-situ falling sphere viscometry is the best method to directly measure viscosity under high-pressure conditions. Based on the Stoke's law (Eq.1), the viscosity of melt is evaluated from the vertical velocity of the sphere, which is monitored through sequential radiographic images (Fig.5a).
| |
・・・ (1) |
where vs, rs, ps, pm and g are the terminal velocity, sphere radius, sphere density, melt density, and gravity acceleration, respectively.
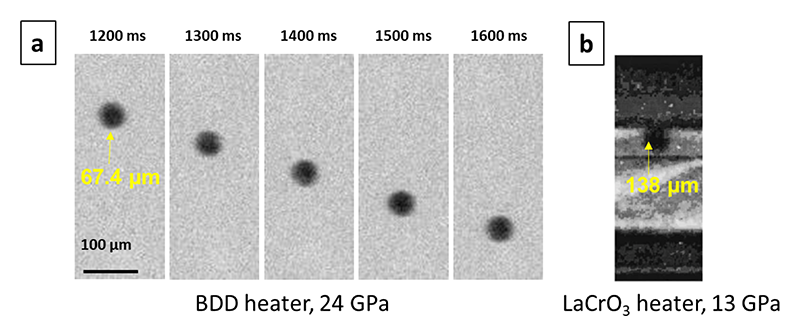
Figure 5 (a) Clear radiographic image of a probe sphere falling in liquid forsterite using a BDD heater. (b) Radiographic image of a probe sphere in LaCrO3 heater (Reid et al. 2003).
Before I started my work, the viscosity measurement of silicate melts was limited to ~13 GPa (upper mantle)[14][14] J. E. Reid et al.: Phys. Earth Planet. Inter. 139 (2003) 45-54. for more than 10 years due to the lack of proper heating element (Fig.5b). Being refractory and highly X-ray transparent, BDD is a perfect heating element to extend the pressure range of viscosity data. Thanks to the application of BDD heater, I succeeded to measure viscosity of forsterite1), enstatite2) and diopside3) composition up to 30 GPa at SPring-8 (Japan) and Synchrotron SOLEIL (France). Then, I applied the new viscosity data set to constrain the crystallization processes of the MO in the early Earth. I concluded that a bridgmanite-enriched layer forms at the top lower-mantle during the cooling of magma ocean (Fig.6). This study was published in Nature Communications (2020)[15][15] L. Xie et al.: Nat. Commun. 11 (2020) 548..
1) Forsterite (Mg2SiO4): the magnesium endmember of olivine, a major mineral of mantle.
2) Enstatite (MgSiO3): the magnesium endmember of pyroxene, a major mineral of mantle.
3) Diopside (CaMgSi2O6): a monoclinic pyroxene mineral.
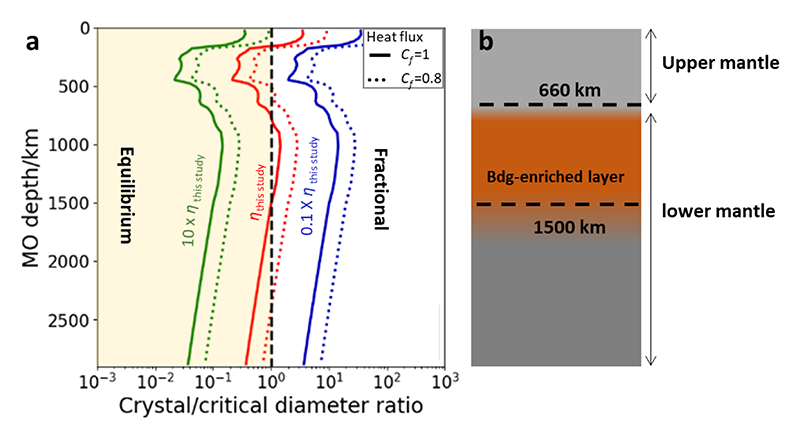
Figure 6 (a) Crystal/critical diameter ratio (Rcc) as a function of magma ocean (MO) depth. Critical diameter is the largest grain size of crystal that can be suspended in a convecting MO. Crystal diameter is the grain size in the MO. Fractional or equilibrium solidification should occur when Rcc is large or smaller than 1, respectively. The heat flux of MO is affected by the atmosphere. When we ignore the blanketing effect of atmosphere (Cf = 1), MO has the highest heat flux, resulting in the smallest Rcc value (solid lines). When we consider the blanketing effect (e.g. 20%, ie Cf = 0.8), MO has a lower heat flux, resulting in a larger Rcc value (dotted lines). In order to show the effect of viscosity on Rcc value, Rcc calculated with 10 and 1/10 times viscosity are also plotted. Even with the highest heat flux, fractional solidification (Rcc > 1) should occur at depth of ~1000 km. (b) A conceptual drawing of mantle after magma ocean solidification. Bdg: bridgmanite.
Acknowledgements
A part of chapter II is a cooperation work with A. Yoneda, E. Ito, T. Yoshino, D. Yamazaki, N. Tsujino at Institute for Planetary Materials (IPM), Okayama University, Japan; Y. Higo, Y. Tange at Japan Synchrotron Radiation Research Institute (JASRI), Japan; and T. Irifune, T. Shimei at Geodynamics Research Center, Ehime University, Japan. B part of chapter II is a cooperation work with A. Yoneda at IPM; F. Xu, D. Antonangeli, G. Morard at Institut de Minéralogie, de Physique des Matériaux, et de Cosmochimie, France; C. Wang at State Key Laboratory of Geological Processes and Mineral Resources, China; Y. Higo, Y. Tange at JASRI; and A. King, N. Guignot at Synchrotron SOLEIL, France. Part III is a collaboration work with A. Yoneda, D. Yamazaki at IPM; D. Andrault, G. Manthilake at Laboratoire Magmas et Volcans, France; Y. Higo, Y. Tange at JASRI; N. Guignot, A. King, M. Scheel at Synchrotron SOLEIL, France. The synchrotron radiation experiments were performed under SPring-8 Budding Researcher Support Program (No. 2015A1771, 2016A1651, 2016B1686, 2017B1686 and 2018A1637) and SOLEIL research proposals (No. 20160333 and 20170194). I thank A. Yoneda and Fang Xu for proof reading.
References
[1] E. Ito: Miner. Phys. 2 (2007) 197-230. (doi: 10.1016/b978-044452748-6/00036-5)
[2] T. Katsura: Spec. Pap. Geol. Soc. Am. 421 (2007) 189-205.
[3] M. Kanzaki et al.: High-Pressure Research in Mineral Physics: A 39 (2011) 195-200. (doi:10.1029/gm039p0195)
[4] T. Sakamaki, E. Ohtani, S. Urakawa, A. Suzuki and Y. Katayama: Earth Planet. Sci. Lett. 287 (2009) 293-297.
[5] A. Yamada et al.: High Press. Res. 28 (2008) 255-264.
[6] A. Shatskiy et al.: Rev. Sci. Instrum. 80 (2009) 023907.
[7] A. Yoneda, L. Xie, N. Tsujino and E. Ito: High Press. Res. 34 (2014) 392-403.
[8] L. Xie, A. Yoneda, T. Yoshino, H. Fei and E. Ito: High Press. Res. 36 (2016) 105-120.
[9] L. Xie et al.: Rev. Sci. Instrum. 88 (2017) 093904.
[10] H. Ozawa et al.: High Press. Res. 38 (2018) 120-135.
[11] L. Xie et al.: Rev. Sci. Instrum. 91 (2020) 043903.
[12] W. B. Tonks and H. J. Melosh: J. Geophys. Res., Planets 98 (1993) 5319-5333.
[13] V. Solomatov: Treatise Geophys. Second Ed. 9 (2015) 81-104.
[14] J. E. Reid et al.: Phys. Earth Planet. Inter. 139 (2003) 45-54.
[15] L. Xie et al.: Nat. Commun. 11 (2020) 548.
*Corresponding author; Tel: +49(0)921 55 3743, Fax: +49(0)921 55 3769, E-mail: ddtuteng@gmail.com








