Volume 14, No.2 Pages 154 - 158
2. 最近の研究から/FROM LATEST RESEARCH
Long-term Proposal Report 3:
IMAGING LUNG AERATION AT BIRTH USING PHASE CONTRAST X-RAY IMAGING
[1]Monash Centre for Synchrotron Science, [2]Department of Physiology, Monash University, [3]School of Physics, Monash University, [4]Division of Neonatology, Department of Pediatrics, Leiden University Medical Centre, [5]Division of Biological Engineering, Monash University, [6]SPring-8/JASRI
Introduction
During fetal life, the lungs are liquid-filled and are not required for gas exchange which occurs across the placenta. However, at birth, the responsibility for gas exchange falls immediately upon the lung (5, 6), but to perform this task the airways must be cleared of liquid to allow the entry of air. The entry of air not only allows the onset of pulmonary gas exchange, but also initiates a major increase in blood flow through the lungs as well as the closure of vascular shunts that allow blood to by-pass the lungs during fetal life. These changes are essential for the survival of the newborn infant (7).
Infants born very preterm (< 28 weeks of gestation) commonly have difficultly initiating and maintaining breathing after birth because their lungs are very immature (17). In particular, the lungs of preterm infants are unable to develop and maintain air within the lungs at the end of expiration. This is largely because they are unable to successfully clear the liquid from their airways which reduces the gas volume of the lung and restricts its ability to exchange gases. As a result, the increase in pulmonary blood flow is also restricted and the vascular shunts that by-pass the lungs do not close. Understanding the factors that promote lung aeration and airway liquid clearance at birth are vital to improving the treatment of babies born very preterm. Preterm birth is a major problem in most societies, occurs in ~10% of all births, and is the leading cause of death and disease in newborn babies (14). At SPring-8, we have developed an imaging technique, called phase contrast (PC) X-ray imaging that can image the lung with high resolution (15). In particular, it has allowed us to investigate the process of lung aeration at birth in spontaneously breathing as well as mechanically ventilated newborn rabbit pups.
Phase contrast (PC) X-ray imaging
Phase contrast X-ray imaging exploits the differences in refractive indices between air and water to greatly enhance image contrast of the air-filled lung; a comparison between normal absorption contrast and phase contrast for imaging a bubble in water is displayed in figure 1. When X-rays pass through the lung, the X-rays are refracted at each boundary between air and water due to the refractive index differences between these media. As the lung is mostly comprised of air (~80% by volume) located within millions of small air sacs that are surrounded by thin ribbons of tissue (mostly comprised of water), PC is particularly useful for imaging the lung (13, 16, 23). Using this technique, the lung is not visible when it is filled with liquid and only becomes visible as it fills with air after birth (Fig. 2) (8). We have used this technique to image newborn rabbit pups to investigate the factors that regulate the rate and pattern of lung aeration after birth. The information obtained is beginning to influence the way in which preterm infants are ventilated immediately after birth in those infants requiring assistance.
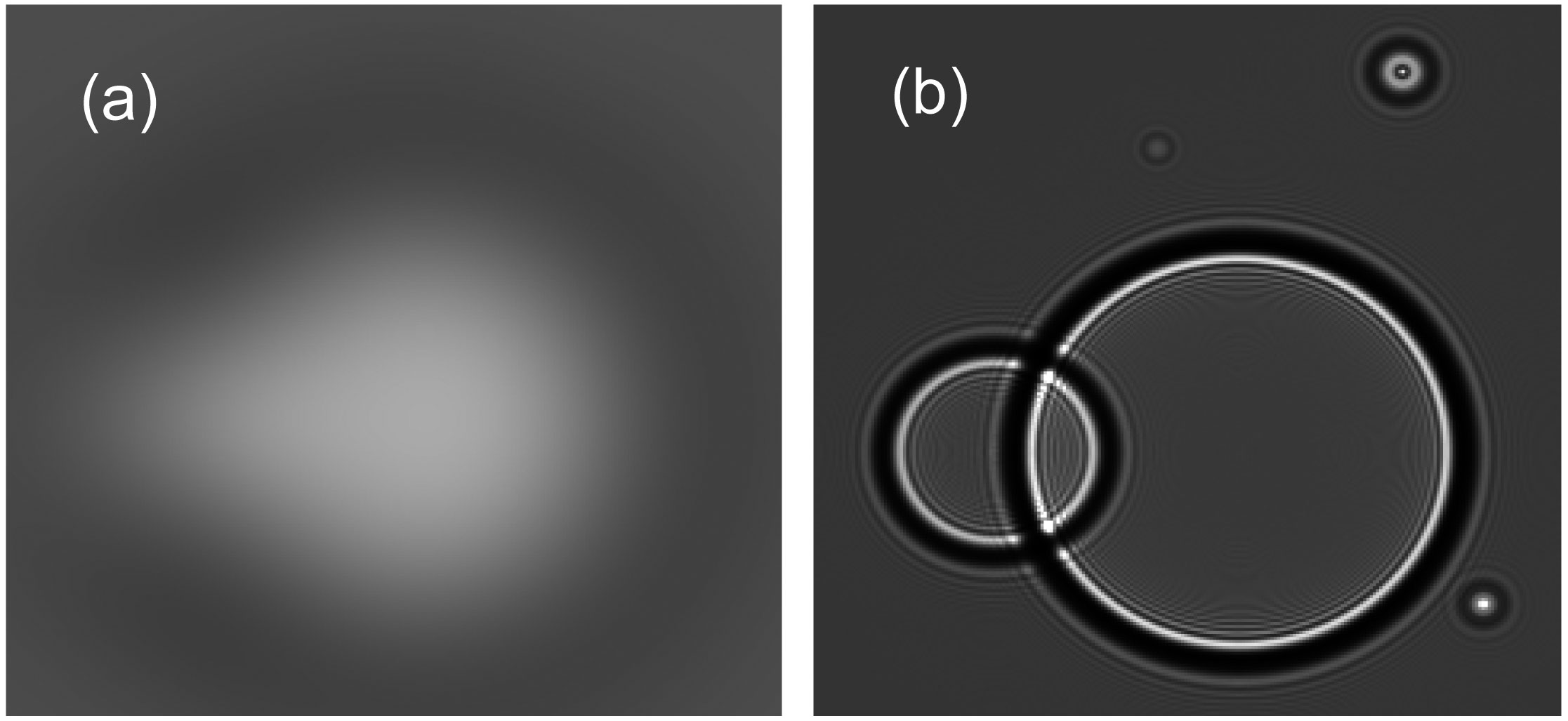
Figure 1 Contrast due to X-ray absorption (a) is unable to clearly resolve the air bubble whereas phase contrast X-ray imaging (b) can clearly resolve the same air bubble.
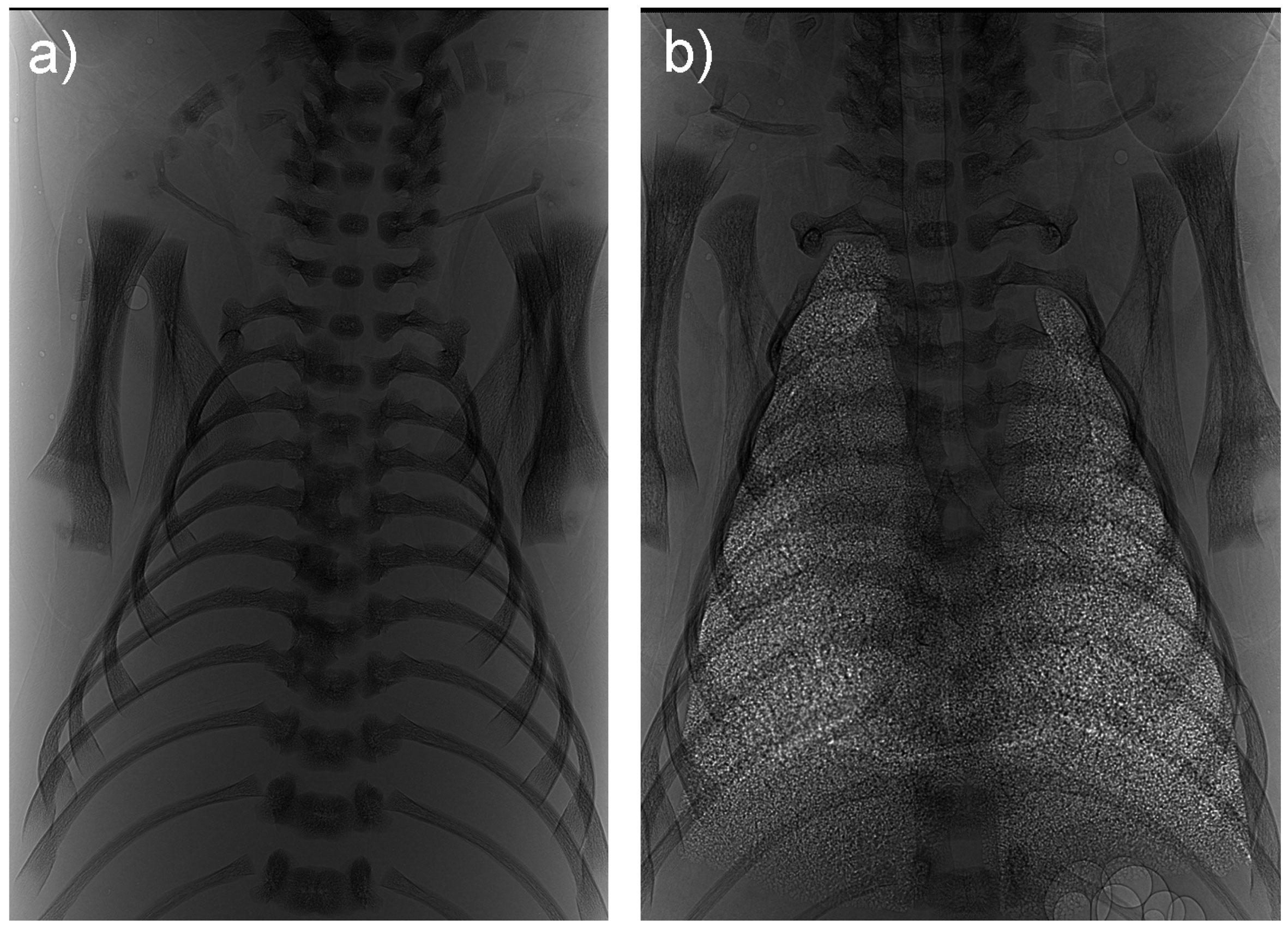
Figure 2 Phase contrast X-ray image of the lungs of a rabbit pups delivered at term and imaged (a) before the onset of lung aeration, when the airways are liquid-filled and (b) 1 hour after the onset of air breathing The trachea and the margins of the lung lobes can also be observed.
Lung liquid clearance at birth
Despite major advances in the care of very preterm infants, these infants continue to suffer from debilitating lung disease (1, 3) which can extend for many months after birth and continue into childhood. As airway liquid retention is a significant cause of respiratory morbidity after birth, the factors regulating airway liquid clearance are very important. The current proposed mechanism for airway liquid removal suggests that increased adrenaline secretion, caused by the stress of labour, activates mechanisms that result in the transport of Na+ out of the airway lumen (9, 18). This creates an osmotic gradient that promotes the movement of water in the same direction (11, 19). However, our recent phase contrast imaging studies, performed at SPring-8, indicate that Na+ reabsorption is unlikely to be the main mechanism responsible for clearing airway liquid after birth. This was demonstrated in both spontaneously breathing term pups as well as mechanically ventilated preterm pups.
Lung liquid clearance and lung aeration in spontaneously breathing newborn rabbit pups
Our findings indicate that the creation of a pressure gradient between the airways and the surrounding lung tissue during inspiration is largely responsible for airway liquid clearance at birth. In rabbit pups delivered at term (31 days of gestation) and allowed to breathe spontaneously, the increase in gas volume of the lung was almost totally associated with inspiration. This was demonstrated by both physiological recordings as well as by the PC X-ray images (Fig. 3). We found that pups inhale more air than they exhale, with the difference in gas volume remaining in the lung at end expiration (Fig. 3). As a result, the gas volume of the lung accumulates with each successive breath allowing some pups to fully aerate their lungs within 5-6 breaths after birth. This is much more rapid than previously considered and ~95% of the total increase in lung gas volume occurred during successive breaths. In contrast, < 5% of the increase in gas volume could be attributed to other non-breathing related mechanisms such as Na+ reabsorption (21).
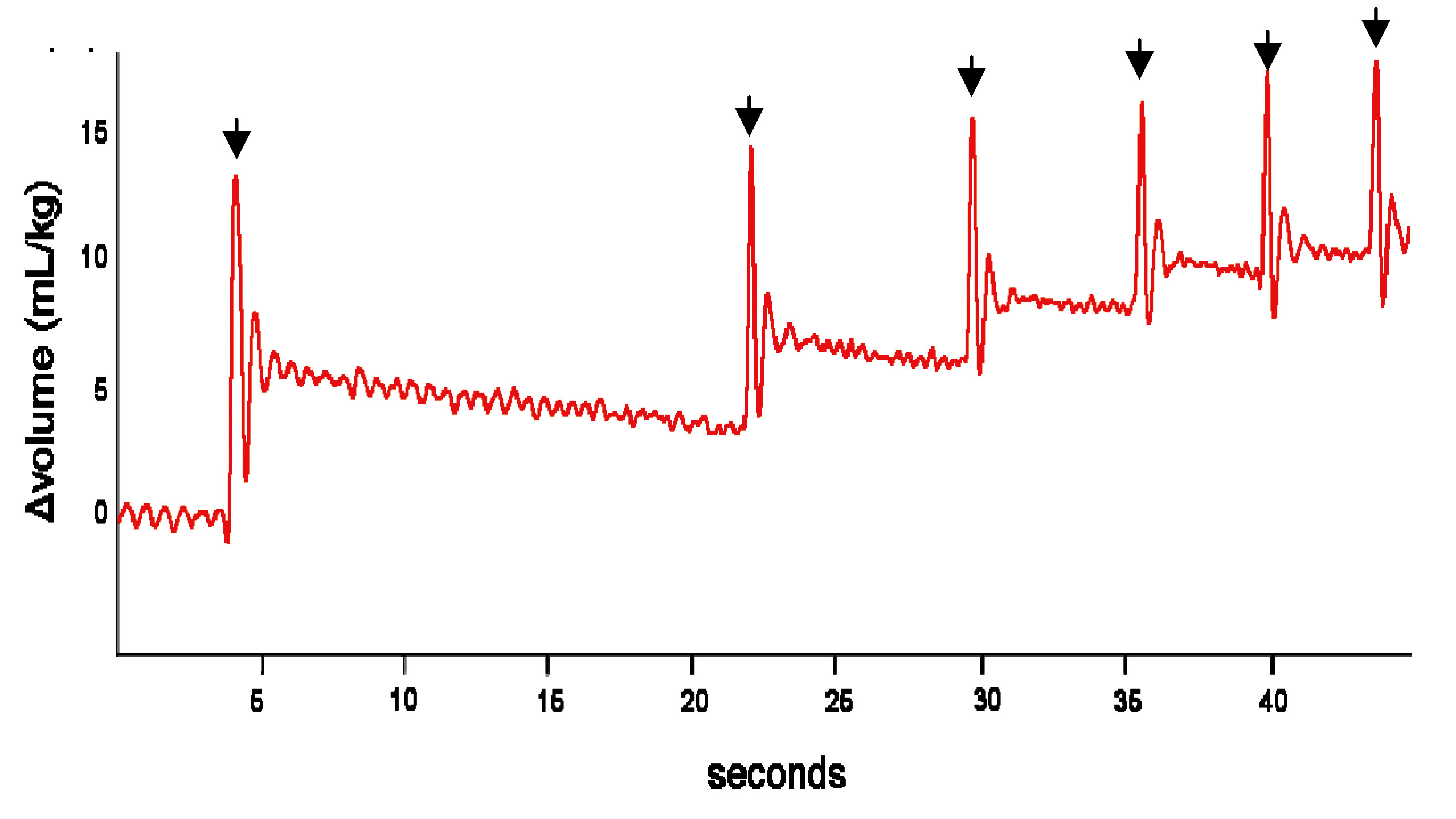
Figure 3 The resting gas volume of the lung increases after birth with each subsequent breath. Individual breaths are indicated by arrows (from ref. 23).
In view of these findings, we have proposed that airway liquid clearance is driven by inspiration which creates a hydrostatic pressure gradient between the airspaces and surrounding lung tissue. Contraction of the diaphragm during inspiration causes the chest to expand which creates a sub-atmospheric pressure in both the lung tissue surrounding the airways as well as in the space between the chest wall and the lung (intrapleural space). This causes the lung to expand, allowing air to enter the airways. Although the pressure in the airways also initially decreases during inspiration, the decrease is not as large as the pressure decrease in the surrounding tissue. As a result, a hydrostatic pressure gradient is created across the airway wall during inspiration which draws water from the airspaces into the lung tissue. Our ability to observe the lungs as they transition from a fluid-filled to an air-filled state using PC X-ray imaging has highlighted the role of this mechanism in airway liquid clearance, which has been largely overlooked previously.
Lung aeration in mechanically ventilated preterm rabbit pups
Preterm newborns are born at a stage of lung development when the lungs are stiff, difficult to inflate, prone to collapse and have difficulty in clearing liquid from their airways. As a result, many of these infants require assistance, usually in the form of mechanical ventilation, from the first breath after birth (4, 12). Mechanical ventilators apply a positive pressure to the airways and thereby force air into the lungs in order to inflate them. However, large regions of the lung can remain fluid-filled and so ventilation can only occur in aerated regions, which exposes those regions to a high risk of injury (2). A major focus of neonatal respiratory research is to develop resuscitation techniques that uniformly aerate the lung and cause minimal damage to it. We have gained considerable insight into the best methods of ventilating very preterm infants using PC X-ray imaging.
Based on our knowledge that airway liquid clearance is determined by the application of a pressure gradient across the airway wall, we have investigated the use of ventilation techniques that enhance this pressure gradient. In particular, we have examined the effect of applying a positive pressure on the airways at end-expiration; this is called PEEP. We found that in the absence of PEEP, pups did not retain air in their lungs following expiration. As a result, the lungs are visible only during inspiration (Fig. 4) and become invisible again at the end of expiration because the airways either re-fill with liquid or collapse; this is known to injure the lung. On the other hand, the application of PEEP caused air to accumulate in the lung with each breath, just as spontaneously breathing pups did when delivered at term (20). Consequently, the lungs are visible throughout the entire respiratory cycle and the distal airways remain aerated even at the end of expiration (Fig. 4). This finding confirms that airway liquid clearance results from a hydrostatic pressure gradient applied across the airway wall and that the application of PEEP is essential for the accumulation of air in the lung at birth in ventilated newborn infants. Currently, PEEP is not recommended for use by the International Liaison Committee on Resuscitation (10), but our imaging studies indicate that this may need re-evaluation.
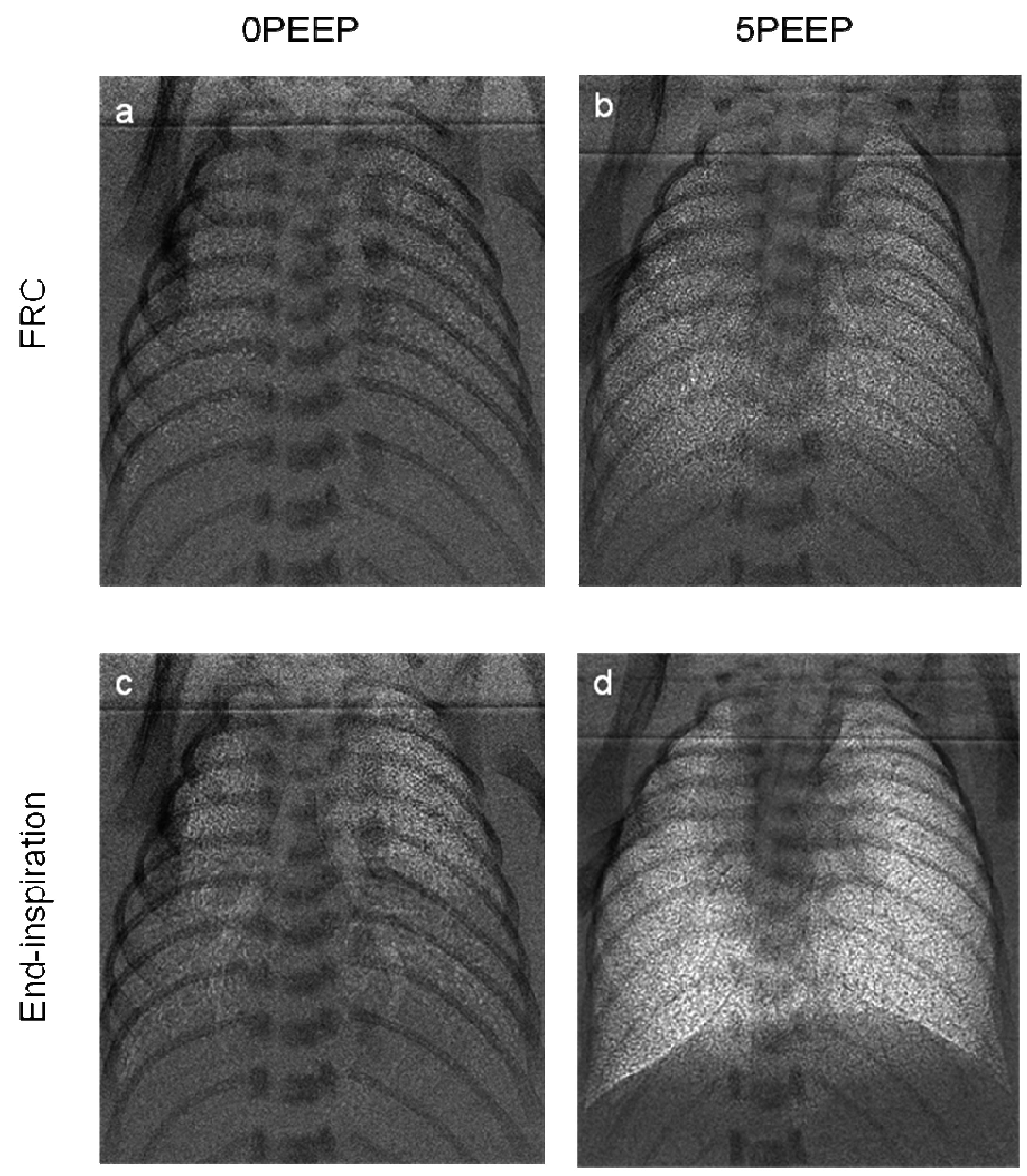
Figure 4 Phase contrast X-ray images of preterm rabbit pups ventilated with (5PEEP; b & d) and without (0PEEP; a & c) a positive end-expiratory pressure (PEEP). Images were acquired at either end-expiration (FRC; a & b) or end-inspiration (c & d) (from ref. 22).
We have also investigated the benefits of applying a sustained (up to 20 secs) inflation of the lung during the first breath after birth. PC X-ray imaging demonstrate that this technique is very successful at uniformly aerating the lung before tidal breathing commences. By applying a prolonged elevation in airway pressure, a sustained inflation maintains a pressure gradient across the airway wall for a prolonged period of time, thereby facilitating liquid clearance from the airways. As this is a time-dependent process, we found that a sustained inflation was very effective at clearing the airways of liquid (22).
Conclusion
In spontaneously breathing newborn rabbits, lung aeration was observed to occur in close association with inspiration, indicating that hydrostatic pressure gradients across the airway wall play an important role in airway liquid clearance at birth. This challenges the current model of airway liquid removal and postulates that hydrostatic pressure gradients are the primary determinant of airway liquid removal after birth. Consistent with this proposal, we have shown that mechanical ventilation using techniques that enhance or sustain the pressure gradient across the airway wall enhance airway liquid clearance after preterm birth. By being able to observe the process of lung aeration using PC X-ray imaging we have been able to greatly advance our understanding of the factors regulating the transition of the lung to air breathing at birth. Such information will greatly improve the techniques used to assist ventilation in very preterm infants who are unable to sustain their respiratory needs after birth.
Reference List
1. Bancalari E, Claure N, Sosenko IR: "Bronchopulmonary dysplasia: changes in pathogenesis, epidemiology and definition" Semin Neonatol 8 63-71, 2003.
2. Bjorklund LJ, Ingimarsson J, Curstedt T, John J, Robertson B, Werner O, Vilstrup CT: "Manual ventilation with a few large breaths at birth compromises the therapeutic effect of subsequent surfactant replacement in immature lambs" Pediatr Res 42 348-355, 1997.
3. Clark RH, Gerstmann DR, Jobe AH, Moffitt ST, Slutsky AS, Yoder BA: "Lung injury in neonates: causes, strategies for prevention, and long- term consequences" J Pediatr 139 478-486, 2001.
4. Gilbert WM, Nesbitt TS, Danielsen B: "The cost of prematurity: Quantification by gestational age and birth weight" Obstet Gynecol 102 488-492, 2003.
5. Harding R, Hooper SB: "Regulation of lung expansion and lung growth before birth" J Appl Physiol 81 209-224, 1996.
6. Hooper SB, Harding R: "Fetal lung liquid: a major determinant of the growth and functional development of the fetal lung" Clin Exp Pharmacol Physiol 22 235-247, 1995.
7. Hooper SB, Harding R: "Role of aeration in the physiological adaptation of the lung to air-breathing at birth" Current Respiratory Medicine Reviews 1 185-195, 2005.
8. Hooper SB, Kitchen MJ, Wallace MJ, Yagi N, Uesugi K, Morgan MJ, Hall C, Siu KK, Williams IM, Siew M, Irvine SC, Pavlov K, Lewis RA: "Imaging lung aeration and lung liquid clearance at birth" FASEB J 21 3329-3337, 2007.
9. Hooper SB, Wallace MJ, Harding R: "Amiloride blocks the inhibition of fetal lung liquid secretion caused by AVP but not by asphyxia" J Appl Physiol 74 111-115, 1993.
10. "International Liaison Committee on Resuscitation. Part 7: Neonatal resuscitation" Resuscitation 67 293-303, 2005.
11. Jain L, Eaton DC: "Physiology of fetal lung fluid clearance and the effect of labor" Semin Perinatol 30 34-43, 2006.
12. Jobe AH, Ikegami M: "Lung development and function in preterm infants in the surfactant treatment era" Annu Rev Physiol 62 825-846, 2000.
13. Kitchen MJ, Paganin D, Lewis RA, Yagi N, Uesugi K, Mudie ST: "On the origin of speckle in x-ray phase contrast images of lung tissue" Phys Med Biol 49 4335-4348, 2004.
14. Laws, P. J. Sullivan, E. A.: Australia's mothers and babies 2003. AIHW Cat. No. PER 29 (Perinatal Statistics No.16). 2005. sydney, AIHW National Perinatal Statistics Unit.
15. Lewis RA: "Medical phase contrast x-ray imaging: current status and future prospects" Phys Med Biol 49 3573-3583, 2004.
16. Lewis RA, Yagi N, Kitchen MJ, Morgan MJ, Paganin D, Siu KK, Pavlov K, Williams I, Uesugi K, Wallace MJ, Hall CJ, Whitley J, Hooper SB: "Dynamic imaging of the lungs using x-ray phase contrast" Phys Med Biol 50 5031-5040, 2005.
17. Moss TJ: "Respiratory consequences of preterm birth" Clin Exp Pharmacol Physiol 33 280-284, 2006.
18. Olver RE, Ramsden CA, Strang LB, Walters DV: "The role of amiloride-blockable sodium transport in adrenaline-induced lung liquid reabsorption in the fetal lamb" J Physiol 376 321-340, 1986.
19. Olver RE, Walters DV, Wilson M: "Developmental regulation of lung liquid transport" Annu Rev Physiol 66 77-101, 2004.
20. Siew ML, te Pas AB, Wallace MJ, Kitchen MJ, Lewis RA, Fouras A, Morley CJ, Davis PG, Yagi N, Uesugi K, Hooper SB: "Positive end expiratory pressure enhances development of a functional residual capacity in preterm rabbits ventilated from birth" J Appl Physiol 2009.
21. Siew ML, Wallace MJ, Kitchen MJ, Lewis RA, Fouras A, te Pas AB, Yagi N, Uesugi K, Siu KK, Hooper SB: "Inspiration regulates the rate and temporal pattern of lung liquid clearance and lung aeration at birth" J Appl Physiol 2009.
22. te Pas AB, Siew M, Wallace MJ, Kitchen MJ, Fouras A, Lewis RA, Yagi N, Uesugi K, Donath S, Davis PG, Morley CJ, Hooper SB: "Establishing functional residual capacity at birth: the effect of sustained inflation and positive end expiratory pressure in a preterm rabbit model" Pediatr Res 2009.
23. Yagi N, Suzuki Y, Umetani K, Kohmura Y, Yamasaki K: "Refraction-enhanced x-ray imaging of mouse lung using synchrotron radiation source" Med Phys 26 2190-2193, 1999.








