Volume5 No.1
SPring-8 Section A: Scientific Research Report
Alteration of Mouse Nasal Airway Surface Mucociliary Transit by Airway Rehydrating Agents
aRespiratory & Sleep Medicine, Women’s and Children’s Hospital, North Adelaide, South Australia
bRobinson Research Institute, University of Adelaide, South Australia
cSchool of Medicine, University of Adelaide, South Australia
dSchool of Physics, Monash University, Victoria, Australia
eMechanical and Aerospace Engineering, Monash University, Clayton, Vic, 3800, Australia
- Abstract
-
Cystic fibrosis (CF) is a genetic disorder that compromises the ability of the mucociliary transit (MCT) system to clear debris and pathogens from the airways. To directly assess airway health and the effects of treatments we have developed a synchrotron X-ray microscopy method that non-invasively measures the local rate and patterns of MCT behaviour. The aim of this experiment was to determine if our non-invasive local airway health assessment method could identify changes in nasal MCT rate following clinical treatments known to alter MCT.
Experiments were performed on the BL20XU beamline at the SPring-8 Synchrotron in Japan. Mice were anaesthetized, a small quantity of lead sulphide particles were delivered to the nose, and images of the nasal airways were acquired. The nasal airways were treated with hypertonic saline or mannitol to increase surface hydration and change MCT. Custom software was used to locate and track the motion of the lead particles throughout the image sequences, and to calculate individual and bulk MCT rates.
MCT rates increased following both treatments, but due to high variability there were no statistically significant differences in MCT rate between treatments. However, in future studies we hope that the improved sensitivity provided by this technique will allow us to identify useful CF lung disease-modifying therapies.
Keywords: Phase contrast, synchrotron, X-ray imaging, mouse, nose, cystic fibrosis, mucociliary transit
Background and Purpose
Cystic fibrosis (CF) is caused by a defective CF transmembrane conductance regulator (CFTR) gene that disrupts ion-transport across the airway epithelium [1]. The resulting airway surface dehydration impairs the ability of the mucociliary transit (MCT) system to clear the airways of debris and pathogens, permitting infections to establish. Lung disease starts early in childhood and continues relentlessly, dramatically reducing quality and length of life.
Until recently there were no methods able to directly and non-invasively measure MCT in vivo. Primary outcome measures typically utilized for CF drug development studies include changes in lung function (FEV1), structural lung imaging (i.e. computed tomography), quantification of the number and severity of pulmonary exacerbations, and quality of life questionnaires. All of these are indirect measures of airway surface hydration, and require long periods of treatment to detect measurable downstream changes.
MCT quantification is traditionally performed using inhaled radio-labelled particles [2, 3], but these methods are relatively insensitive and are unsuited to topographically-complex airways. We have developed a MCT quantification method that tracks the motion of deposited particles using synchrotron X-ray microscopy [4, 5]. This allows us to rapidly and non-invasively measure MCT behavior in vivo to directly characterize airway health and the efficacy of treatments. Our setup uses propagation-based phase contrast X-ray imaging (PCXI), which exploits the refractive properties of X-rays to provide enhanced image contrast in addition to conventional absorption contrast. With this setup we achieve a magnification / resolution at least two orders of magnitude higher than other current methods such as μ-CT and MRI [6]. Since the air-tissue interfaces are enhanced by these phase effects PCXI is particularly useful for imaging the soft-tissues lining the nose of live anaesthetized mice. The technique works best with extremely high flux, coherent, monochromatic X-rays that can currently only be produced using a synchrotron, with optimum conditions present at long beamlines such as BL20XU at the SPring-8 Synchrotron. The enhanced airway surface visibility is well above that of conventional X-ray imaging, allowing for unique detailed measurements of airway surface MCT activity in live mice.
Inhaled hypertonic saline (HS) [7-9] and inhaled mannitol [10] are two current clinical respiratory treatments used in CF for their osmotic properties. Both slow the progression of lung disease by drawing water onto the airway surface and rehydrating the airway surface liquid layer to improve mucociliary function and the clearance of mucus. As a small ionic species, deposited HS (7% sodium chloride, MW 58) undergoes rapid homeostasis via epithelial cell ion-channels and tight junction control of water movement in normal airways. In contrast, mannitol is a large sugar alcohol molecule (MW 182), for which there is no homeostatic or absorptive cellular control mechanisms present on the airway surface. For this reason we hypothesized that the effect of mannitol would significantly outlast that of HS.
The aim of this experiment was to determine if our non-invasive airway health assessment method could identify differences in nasal MCT rate between these two common clinical airway rehydration therapies, potentially lost in traditional bulk MCT measurements.
Experimental Summary
Experiments were performed on the BL20XU beamline at the SPring-8 Synchrotron, under approvals from the Animal Ethics Committees of SPring-8, the Women’s and Children’s Hospital, and the University of Adelaide.
The imaging setup was as previously described [4, 11]. Briefly, imaging was performed in the downstream experimental hutch 245 meters from the storage ring, using 25 keV monochromatic X-rays and a sample-to-detector distance of ~80 cm. The image field of view was 1.2 mm x 1.43 mm (2160 x 2560 pixels). Exposure times of 10 ms were used to produce a high SNR without movement blur.
Mice (n=17 normal C57Bl/6) were prepared for imaging as described previously [4, 11]. Mice were anaesthetized with sodium pentobarbital, and the fur around the imaging area was removed using clippers and a depilatory cream to minimize phase artefacts in the images. A small quantity of lead sulphide dust (less than 0.001 g PbS) was insufflated into the right nasal airway using a Dry Powder Insufflator™ (PennCentury, USA). Mice were secured in an imaging holder and placed so that the X-ray beam passed dorso-ventrally through the tip of the nose. Baseline images were acquired at 2 Hz for one minute. The right nasal airway was then treated with either 60 seconds of 7% hypertonic saline or 9% mannitol aerosol delivered using an Aeroneb nebulizer (Aerogen, Ireland) mounted above the nose such that the mice passively inhaled aerosol during each breath. Image acquisition continued at 2 Hz for a further 20 minutes.
Images were flat-field corrected, and custom software [4] was used to track particle motion in a semi-automated manner, and to calculate individual and bulk MCT rates. The quantitative analysis tracked all the moving particles for 20 frames over 12 time points that included baseline (t=-1) and during aerosol delivery (t=0). The MCT rate at each time point in each animal was calculated by averaging all of the instantaneous MCT rates for all the moving particles over the 20 frames at that time point. As in previous experiments, particles moving faster than 15 mm/min were excluded from the analysis because their motion likely resulted from rapid bulk fluid movements. Statistical analyses (2-way ANOVA with Bonferroni multiple comparisons) were performed using GraphPad Prism 5.
Results
Five mice were excluded from the analysis due to the presence of too much lead dust or too much confounding animal movement to enable accurate MCT tracking, leaving n=6 per treatment group. An initial qualitative analysis of the image sequences suggested that in many of the HS treated animals there was a rapid but moderate increase in nasal MCT rate, typically beginning within 10 seconds post HS aerosol delivery, and lasting ~120 seconds. In the mannitol treated group the increases in MCT rate were initially smaller and slower (starting ~90 seconds after delivery), but lasted longer than HS.
Fig. 1: (a) The number of tracked particles at each time point was strongly skewed to the first 5 minutes of imaging. No moving particles were detected at 15 or 20 minutes post-treatment in any animal in either group. (b) The measured MCT rate at each time point.
The quantitative analysis showed that there was one non-responder in each group, which may have been due to an aerosol delivery failure or misplacement of the Aeroneb that resulted in poor aerosol delivery to the mouse nose. The maximum number of particles tracked at any time point was 33 in the HS group (3.5±7.0) and 49 in the mannitol group (6.5±11.0). More moving particles were tracked in the mannitol group at all time points,including at baseline. The presence of moving particles was strongly skewed to the first ~5 minutes of the experiment, with relatively few moving particles tracked after 5 minutes, reducing the reliability of the MCT rate measurements at the later time points (see Fig. 1a). Figure 1b shows that there was an increase in MCT rate compared to baseline after either treatment, and this effect lasted ~5 minutes. Mannitol typically produced larger increases in MCT rate, and although there appeared to be some measured differences in MCT rate between the two treatments none were statistically significant.
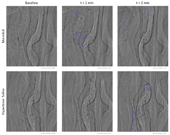
Figure 2 shows examples of the tracking of moving lead particles in the mannitol and HS treatment groups, at baseline and two time points post-delivery.
Fig. 2: Insufflated lead sulphide MCT marker particles are visible in synchrotron PCXI images of mouse nasal airways. The initial location of each tracked particle is identified with a red dot, with the location of that same particle in subsequent frames marked with blue dots. Stationary particles are not marked. Images are 1.2 mm x 1.43 mm.
Discussion
We have demonstrated that MCT can be visualized by tracking the motion of deposited lead sulphide dust along the nasal airway surface, although no statistically significant differences were found between the mannitol and HS treatment groups. This is most likely because the study was poorly powered (see Challenges below), but may also indicate there are no differences between the two treatments, or that our method lacks the sensitivity to detect differences. In any case, further studies are required.
Until this work it was not possible to non-invasively assess the effectiveness of potential treatments at their site of action on live airway surfaces. With this new methodology we plan to determine the efficacy of other clinically relevant airway rehydrating therapies in the mouse nose and trachea, as well as using alternative models of CF–like lung disease (β-ENaC mice). We expect that the improved sensitivity available with this technique will accelerate the ability to test useful CF lung disease-modifying interventions in small animal models, and enhance the development of proposed new therapies.
Challenges
Lead sulphide is likely to be an irritant in the mouse nose, and may contribute to coughing and other motion, despite the animal being well anaesthetized throughout the experiment. We hypothesise that the surface properties of the lead dust, in particular its surface irregularity, and the variability in the size of the particles may also affect clearance rates, and increase variability. Particle dosing levels between animals were inconsistent, being too low in some animals and too high in others. In addition, during particle delivery it was not possible to uniformly distribute the particles across the airway surface; as a result, some of the particles cluster together and likely alter the measured MCT rates. The volume of aerosol delivered was also variable and often too high, since we saw particles moving in bulk fluid in many animals. Combined, these factors resulted in movement artefacts that made particle tracking much more challenging and reduced the accuracy of the MCT rate measurements, as well as requiring removal of some animal data from the analysis. With too much liquid it is also difficult to separate true MCT from particles in bulk fluid flow. Remotely and accurately delivering small quantities of the marker particles and the two therapies to the nasal airways is a major challenge, and we are developing improved methods of achieving this. The experiment was not sufficiently powered to differentiate the differences between the two treatments. In future experiments we will use larger group sizes (power analyses suggests n=14 per group), reduce the amount of aerosol delivered (while increasing the aerosol delivery length), improve the particle delivery method, and also test the effectiveness of more uniform MCT marker particles.
Acknowledgements
Studies supported by the WCH Foundation, NH&MRC Australia, philanthropic donors via the Cure4CF Foundation Ltd (www.cure4cf.org), and Pharmaxis Ltd. The synchrotron radiation experiments were performed on the BL20XU beamline at the SPring-8 Synchrotron, with the approval of the Japan Synchrotron Radiation Institute (JASRI) under proposal number 2012B1813. We thank Naoto Yagi, Kentaro Uesugi, Yoshio Suzuki and Akihisa Takeuchi for their assistance at the BL20XU beamline. Dr Wolfgang Jarolimek (Pharmaxis Ltd) provided the mannitol powder. MD is supported by a MS McLeod Postdoctoral Fellowship, KM by an ARC DECRA and VESKI VPRF Fellowship, and AF by an NHMRC CDF. Travel was supported by the Australian Synchrotron International Synchrotron Access Program (ISAP). The ISAP is an initiative of the Australian Government being conducted as part of the NCRIS.
References
[1] J. J. Wine, "The genesis of cystic fibrosis lung disease", J. Clin. Invest., 103, 309-312, (1999).
[2] B. R. Grubb, J. H. Jones, and R. C. Boucher, "Mucociliary transport determined by in vivo microdialysis in the airways of normal and CF mice", American journal of physiology. Lung cellular and molecular physiology, 286, L588-595, (2004).
[3] M. F. Quinlan, S. D. Salman, D. L. Swift, H. N. Wagner, Jr., and D. F. Proctor, "Measurement of mucociliary function in man", Am. Rev. Respir. Dis., 99, 13-23, (1969).
[4] M. Donnelley, K. S. Morgan, K. K. Siu, A. Fouras, N. R. Farrow, R. P. Carnibella, et al., "Tracking extended mucociliary transport activity of individual deposited particles: longitudinal synchrotron X-ray imaging in live mice", J. Synchrotron Radiat., 21, 768-773, (2014).
[5] M. Donnelley, K. S. Morgan, K. K. Siu, and D. W. Parsons, "Dry deposition of pollutant and marker particles onto live mouse airway surfaces enhances monitoring of individual particle mucociliary transit behaviour", J. Synchrotron Radiat., 19, 551-558, (2012).
[6] A. R. Martin, R. B. Thompson, and W. H. Finlay, "MRI measurement of regional lung deposition in mice exposed nose-only to nebulized superparamagnetic iron oxide nanoparticles", J. Aerosol Med. Pulm. Drug Deliv., 21, 335-342, (2008).
[7] E. Daviskas, S. D. Anderson, I. Gonda, S. Eberl, S. Meikle, J. P. Seale, et al., "Inhalation of hypertonic saline aerosol enhances mucociliary clearance in asthmatic and healthy subjects", Eur. Respir. J., 9, 725-732, (1996).
[8] S. H. Donaldson, W. D. Bennett, K. L. Zeman, M. R. Knowles, R. Tarran, and R. C. Boucher, "Mucusclearance and lung function in cystic fibrosis with hypertonic saline", N. Engl. J. Med., 354, 241-250, (2006).
[9] M. R. Elkins, M. Robinson, B. R. Rose, C. Harbour, C. P. Moriarty, G. B. Marks, et al., "A controlled trial of long-term inhaled hypertonic saline in patients with cystic fibrosis", N. Engl. J. Med., 354, 229-240, (2006).
[10] M. L. Aitken, G. Bellon, K. De Boeck, P. A. Flume, H. G. Fox, D. E. Geller, et al., "Long-term inhaled dry powder mannitol in cystic fibrosis: an international randomized study", Am. J. Respir. Crit. Care Med., 185, 645-652, (2012).
[11] M. Donnelley, K. Morgan, N. Farrow, K. Siu, and D. Parsons, "Non-invasive airway health measurement using synchrotron x-ray microscopy of high refractive index glass microbeads", AIP Conference Proceedings, 1696, 020011, (2016).
ⒸJASRI
(Received: May 3, 2016; Early edition: August 25, 2016; Accepted: December 12, 2016; Published: January 31, 2017)