Volume8 No.1
SPring-8 Section A: Scientific Research Report
X-ray Tomography of Echinoid Functional Biomineralized Materials
aUniversity of Copenhagen, bTechnical University of Denmark
- Abstract
-
We have used X-ray nanotomography to image spines of three different sea urchin species to complement previously acquired microtomography data and study the hierachical structure of the spines.
Keywords: X-ray nanotomography, sea urchins, biotemplating
Background and Purpose
All over the animal kingdom, biomineralization is employed to form durable yet lightweight materials such as bones, teeth and mollusk shells. Studying the internal structure of these materials can give us an important insight into how these materials are formed and how we can use nature’s design strategies to improve man-made materials. For this experiment, we focused on the micro- and nanostructure of spines of three different sea urchin species from different habitats and thus different adaptations to their environment: i) the edible sea urchin (Echinus esculentes) and ii) the heart urchin (Echinocardium cordatum) and iii) the purple sea urchin (Sphaerechinus granularis).
Fig. 1. Shell with spines of Echinus esculentes
The heart urchin is a burrow dwelling animal with its spines aligned along its body so they are not expected to carry a load. On the contrary, the spines of the edible and purple sea urchin have evolved as protection against predators and loading would thus be compressive along the spine axis. Before this experiment, we collected X-ray microtomography data with a resolution of ~1 µm on a laboratory source for spines from the three species. In the case of the heart urchin, we also imaged the shell to determine its micromechanical properties using finite element simulations on the recorded tomography data [1]. In order to be able to perform similar mechanical studies for the spines and further develop the model, we wanted to see if there is another layer of structure on a lower length scale (submicrometer) as it can be found in many biomineralized materials.
Experimental Summary
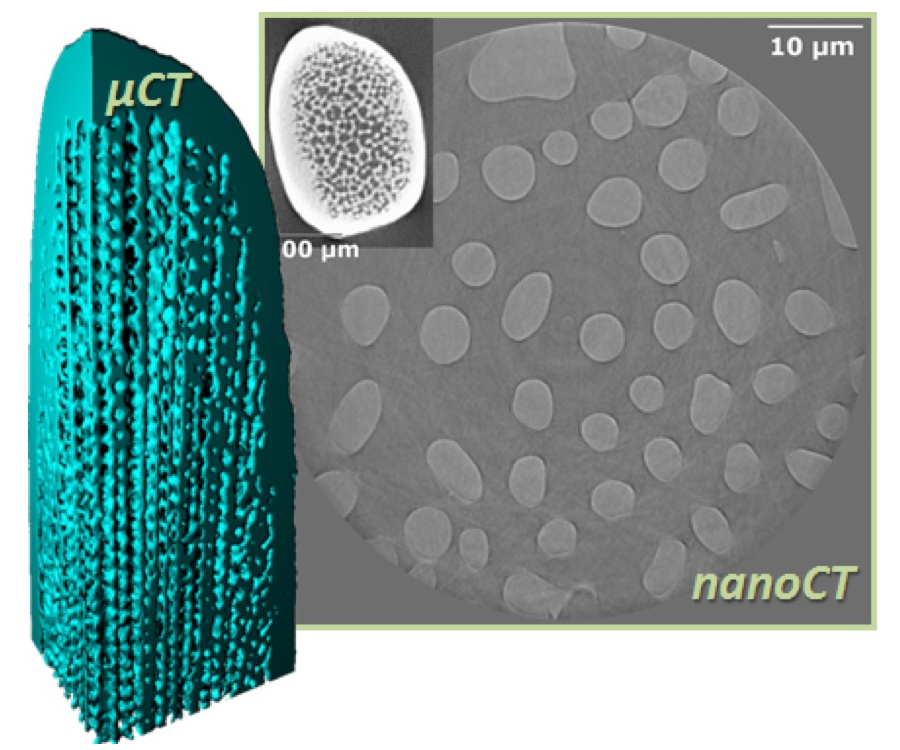
Using the nanotomography setup at beamline BL47XU, we recorded data for seven samples of E. cordatum and S. granularis each and six samples of E. esculentes. For the samples, spines were removed from the shell and mounted on brass pins with grease. The samples were used as received, i.e. no further cleaning steps were performed. Using an X-ray energy of 8 keV, we achieved a voxel size of ~60 nm with an exposure time of 250 ms and 1800 projections. The data were corrected for dark current and beam non-ideality. An example slice of the recorded data for a spine of E. esculentes can be seen in Figure 2 together with the previously recorded microtomography data.
Fig. 2. Microtomography data (3D model on the left and inset to the right) of a spine of E. esculentes together with a slice of nanotomography data from this beamtime showing the underlying structure.
Results and Discussion
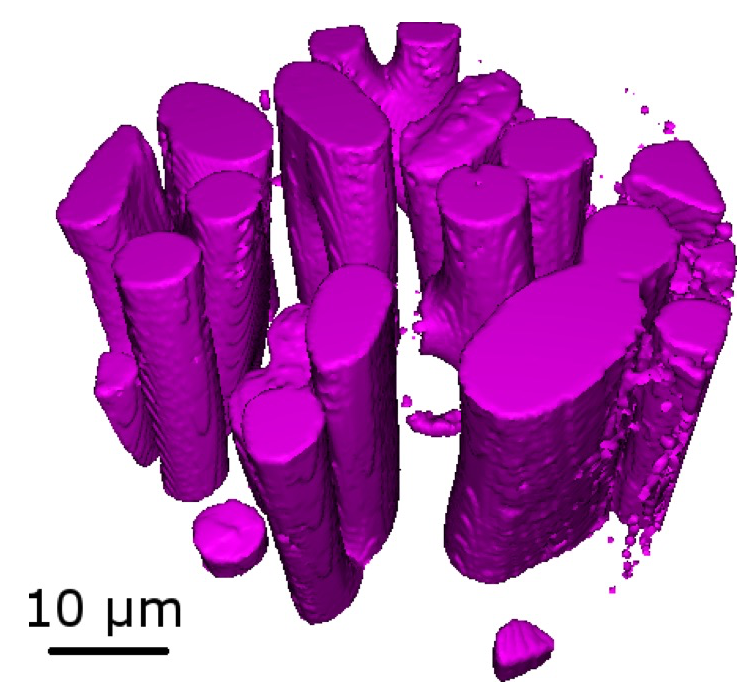
For the heart urchin, we found that the spines are indeed hollow as our previously recorded microtomography data indicated. For the spines of the edible and purple sea urchin, we found that the center of the spines is composed of parallel bundles of struts aligned with the axis of the spines. The struts are periodically interconnected or locally joined (Figure 3). We can interpret this design as an adaptation to the spine’s primary function: protection against predators. The force of an attacking predator would likely be directed along the spine’s axis. Thus, aligning the microstructure (struts) in the same direction maximizes the spine’s stiffness along that axis. The local connections between the spines prevent buckling and take up shear stresses. This design is quite ingenious as it uses a minimum of material, i.e. the central region of the spine has a porosity of ~65%. This minimizes the resources the animal needs to invest while protecting the shell efficiently.
Fig. 3. 3D reconstruction of the central region of a spine of S. granularis showing parallel interconnected struts.
The nanotomography data we recorded in this experiment thus confirm our findings from the microtomography data and show that our mechanical model used in [1] is valid. Additionally, we find that there is no further structural layer on a lower length scale, i.e. the struts appear solid in the nanotomography data and do not contain additional porosity. This is in itself interesting as many other biomineralized tissues show a much more hierarchical structure with morphological changes over a range of length scales [2]. Nanotomography of bone [3], for example, has shown that the osteons are connected by a nanoscale network which could be clearly distinguished at a voxel size of 60 nm. The fact that we cannot see any nanoscale porosity in our data indicates that a similar network does not exist in sea urchin spines, at least not on the same length scale. However, it cannot be ruled out that an underlying ultrastructure exists on an even lower length scale. This could be investigated in 2D using transmission electron microscopy (TEM) or in 3D using a scanning electron microscope with a focused ion beam attachment (FIB-SEM).
Challenges
The 3D images retrieved in this experiment clearly demonstrated that there is no further porosity on the nanoscale inside the struts other than what can be seen in the microtomography data. Thus, this beamtime has validated our previously recorded microtomography data to be sufficient for further studies of sea urchin spines.
References
[1] D. Müter et al., Acta Biomaterialia 23, 21-26 (2015).
[2] H. Ehrlich, Biological Materials of Marine Origin, Springer Science + Business Media B. V. (2010).
[3] M. Langer, A. Pacureanu, H. Suhonen, Q. Grimal, P. Cloetens, F. Peyrin, PLoS ONE 7, e35691 (2012).
(Received: September 9, 2019; Early edition: November 28, 2019; Accepted: December 16, 2019; Published: January 22, 2020)